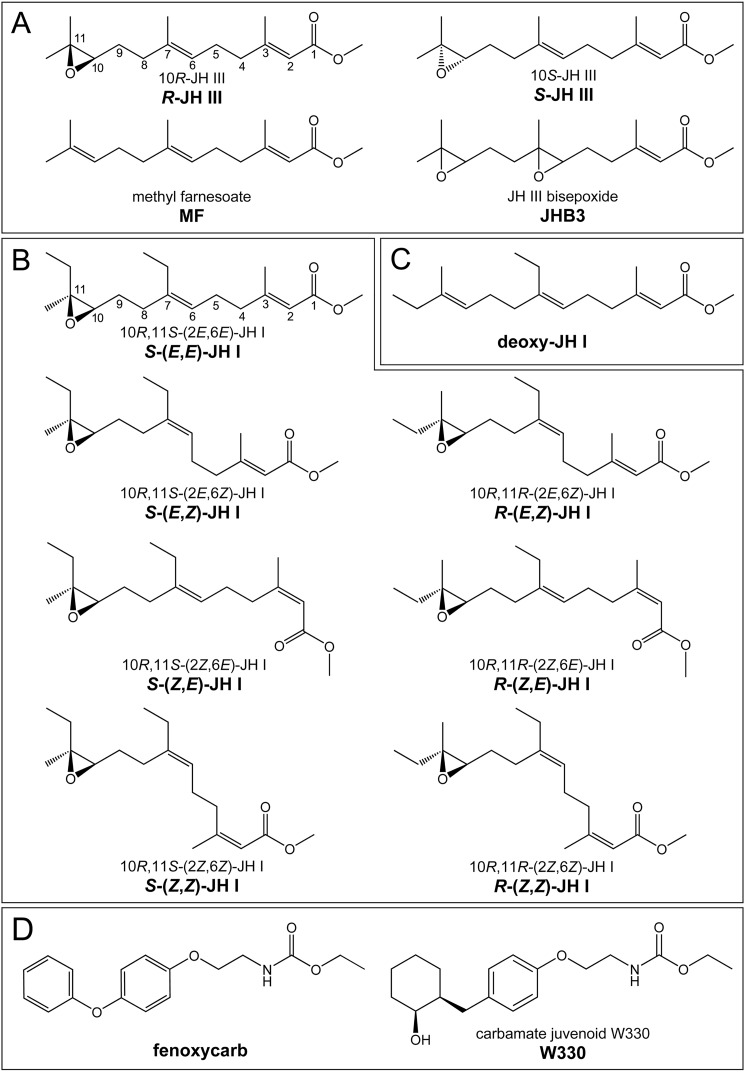
Figure 1.
Structures and names of the tested compounds. A, three known native juvenile hormones in D. melanogaster and the unnatural 10S-JH III enantiomer. B, the native conformation of JH I (top left) and its geometric stereoisomers in both 10R,11S and 10R,11R configurations. C, methyl (2E,6E,10E)-7-ethyl-3,11-dimethyltrideca-2,6,10-trienoate, a nonepoxidated version of JH I, abbreviated as deoxy-JH I. D, carbamate-derived JH mimics. The abbreviated nomenclature (in bold) is preferentially used throughout this study.