Figure 3.
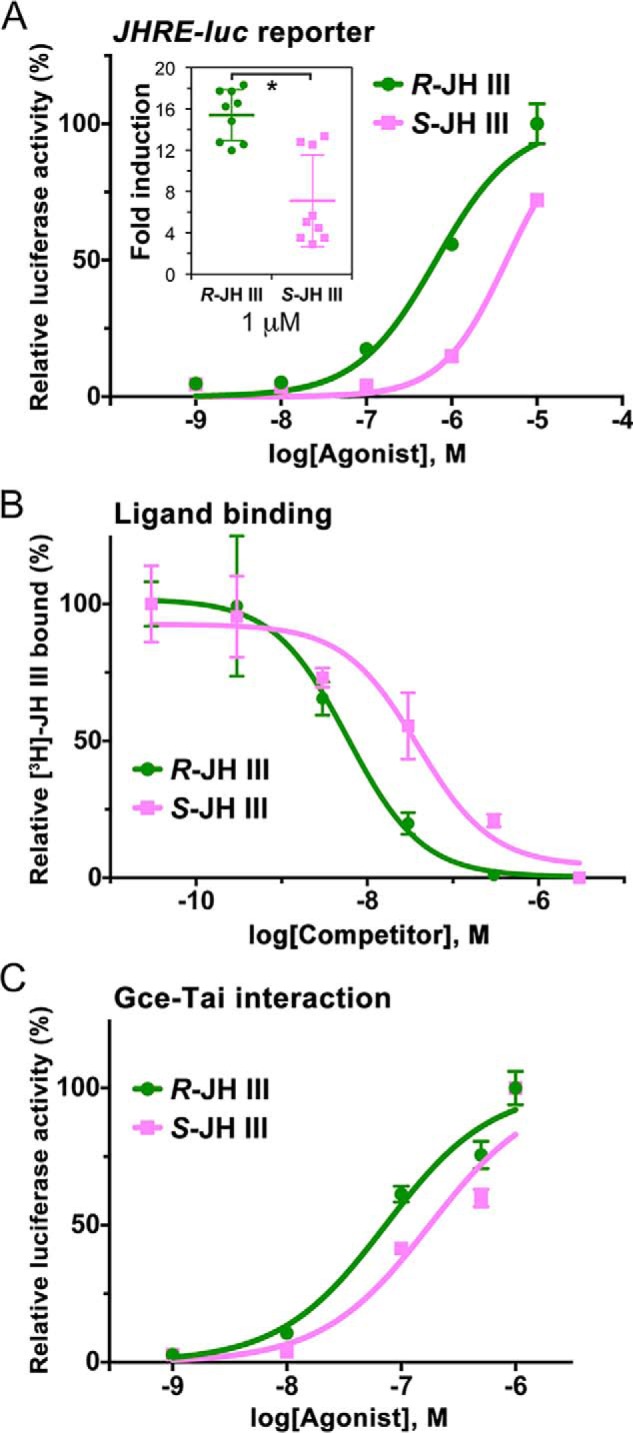
Agonist activities of JH III enantiomers. A, activation of the JH-inducible JHRE-luc reporter in the Drosophila S2 cell line. The data (mean from three technical replicates) show a representative example of three independent experiments. Although calculated EC50 values (0.93 ± 0.38 μm for R-JH III and 4.46 ± 2.95 μm for S-JH III) indicated about a 4-fold lower activity of the latter enantiomer, the difference was not statistically significant. Comparison of JHRE-luc expression at 1 μm concentration (inset) revealed a 2.2-fold higher activity of R-JH III (*, p < 0.05, t test). The normalized luciferase activities are plotted as -fold increase relative to treatment with solvent alone for which the value is arbitrarily set to 1. B, binding of R-JH III and S-JH III to the Gce protein in vitro as assessed from competition against racemic R,S-[3H]JH III. The data (mean from three measurements for each compound) revealed binding affinities (Ki values) of 4.8 ± 1.3 and 38.3 ± 5.2 nm (p < 0.001, t test) for R-JH III and S-JH III, respectively. C, ligand binding–dependent interaction of Gce and Tai components of the JH receptor complex as assessed in a two-hybrid assay in HEK293T cells. The data (mean from three measurements) indicate EC50 values of 73.1 ± 5.2 and 169.8 ± 5.5 nm (p < 0.001, t test) for R-JH III and S-JH III, respectively. Error bars represent S.D. in all panels.
