Figure 4.
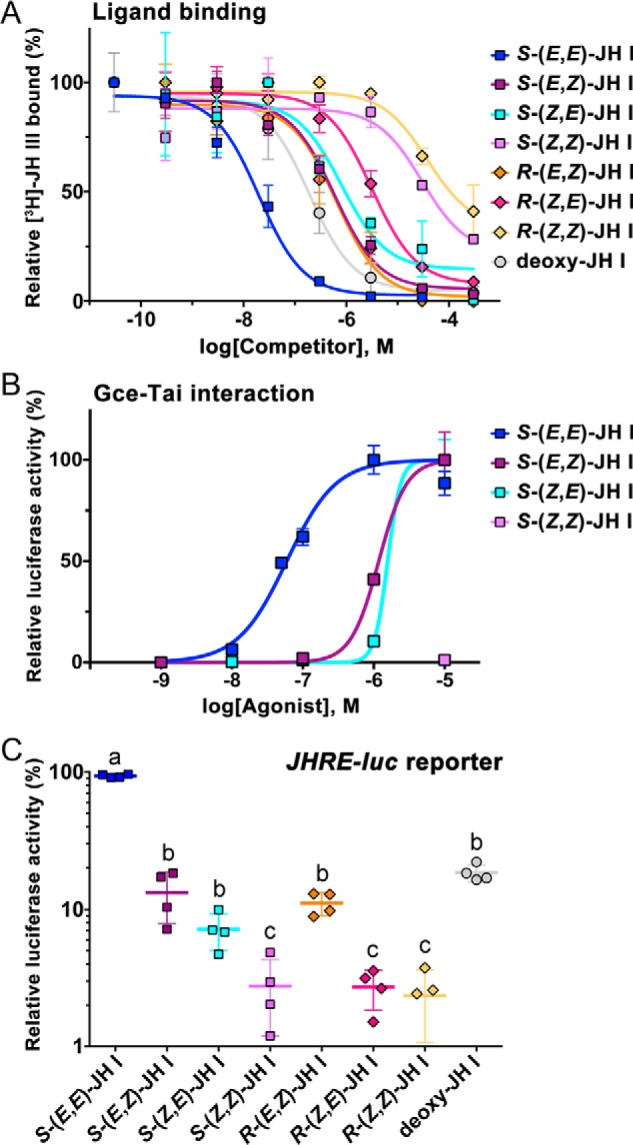
Agonist activities of geometric isomers of JH I. A, binding of the native S-(E,E)-JH I, its stereoisomers, and nonepoxidated deoxy-JH I to the Gce protein in vitro as assessed from competition against R,S-[3H]JH III. The data (mean from three measurements for each compound) revealed the binding affinities (Ki values) listed in Table 1. B, ligand binding–dependent Gce–Tai dimerization in the two-hybrid assay in HEK293T cells. The data (mean from three technical replicates) show a representative example of three independent experiments that together indicated EC50 values of 53.4 ± 13.9 nm for S-(E,E), 1.24 ± 0.09 μm for S-(E,Z), and 1.60 ± 0.04 μm for S-(Z,E) JH I isomers; S-(Z,Z)-JH I was inactive. C, induction of JHRE-luc in the Drosophila S2 cell-based reporter assay by the indicated compounds at 1 μm concentration. The data are mean values from four independent experiments (each in three technical replicates). Different letters above the data indicate that activity of the individual compounds differed significantly (p < 0.05) as determined by one-way analysis of variance of log10-transformed data with Tukey's multiple post hoc comparison. Error bars represent S.D. in all panels.
