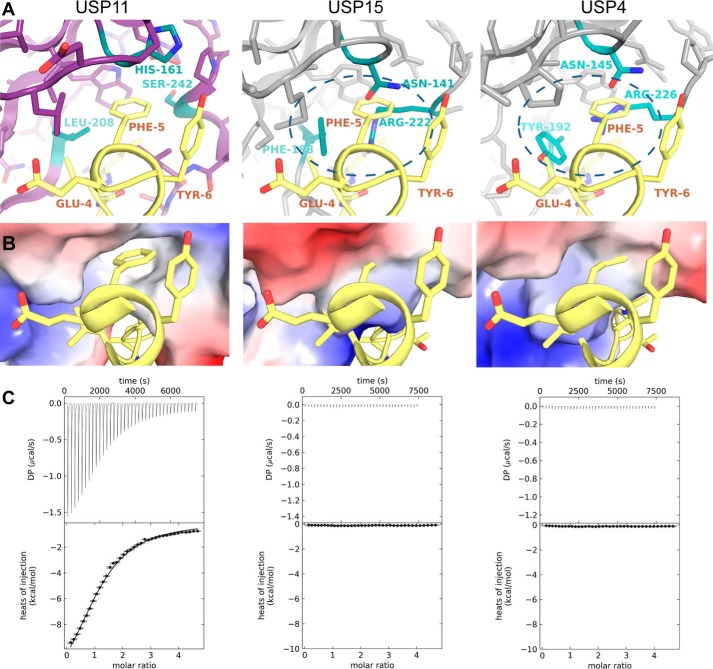
Figure 4.
Molecular basis of FYLIR peptide USP11 specificity. A, close-up views of FYLIR peptide binding to the “major pocket” in the USP11 UBL domain (left panel) compared with USP15 (center panel) and USP4 (right panel), where steric clashes occur when the peptide is modeled into the same position (highlighted by a dashed ellipse). Key residues involved in the interaction (USP11) or preventing peptide binding (USP15 and USP4) are depicted in cyan and labeled. B, close-up views of electrostatic surface representations of FYLIR peptide binding to the major pocket in the USP11 UBL domain (left panel) compared with USP15 (center panel) and USP4 (right panel), where the binding pocket is occluded. C, ITC data of FYLIR peptide with USP11_DU, USP4_DU, and USP15_DU, showing that peptide-ligand binding is highly specific for USP11.