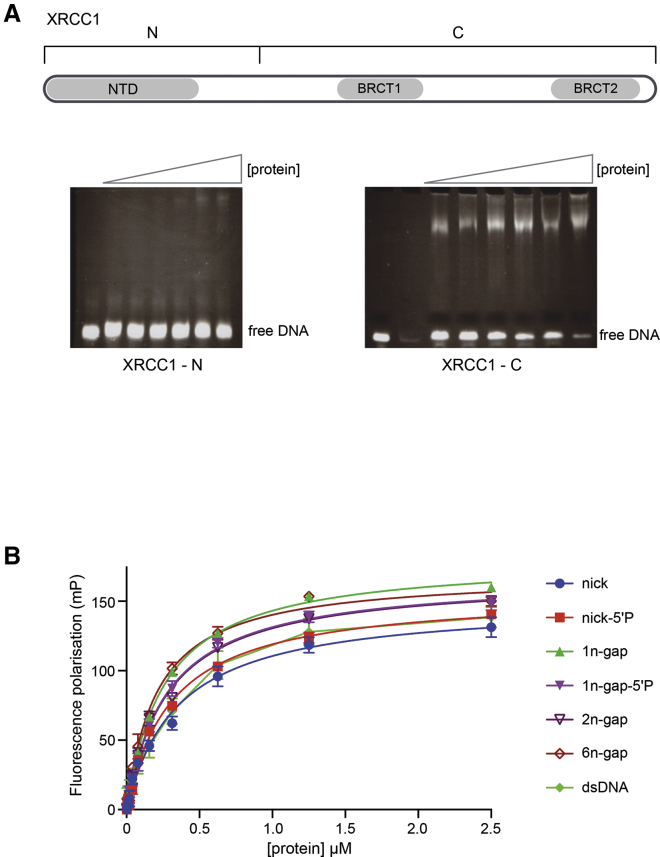
Figure 1.
XRCC1-BRCT1 Binds DNA
(A) Electromobility shift assay (EMSA) shows that the ability to bind DNA resides in the C-terminal region of XRCC1 containing the two BRCT domains rather than the N-terminal region as previously suggested (Marintchev et al., 1999).
(B) Fluorescence polarization assay of XRCC1-BRCT1 binding to various fluorescein isothiocyanate (FITC)-labeled dsDNA oligonucleotides. No substantial differences in affinity were observed between intact, nicked, and gapped molecules, which all bound with sub-micromolar affinity. Oligonucleotide structures and Kd values for their binding to XRCC1-BRCT1 are shown in Figure S1A. Data represent the mean of four measurements comprised of two separate replicates with XRCC1-BRCT1 from two separate protein purifications. Error bars show ± 1 standard error of the mean (SEM).