Abstract
Prostate cancer is one of the most common urological malignancies managed by a practicing urologist. Treatment strategies are varied, but radical prostatectomy (RP) remains a viable and commonly used option for many patients. A continuing challenge in the management is how to approach a patient who has biochemical recurrence (BCR) after RP. There are no consensus guidelines on the appropriate strategy, and the current recommendations, although useful, are at times confusing. The natural history of BCR is heterogeneous. Published studies aid in the clinician's ability to predict patients most likely to recur; however, this remains inexact. In addition, recent changes in the recommendations for disease screening, as well as technological advances, have added to the already challenging task of the clinician. The objective of this review is to provide an up-to-date summary of the definitions, diagnosis, and management strategies of BCR after RP. This narrative review was conducted by searching Medline for all relevant articles in English with the key terms of biochemical recurrence, prostate cancer, management, and other relevant terms. Information was compiled and reviewed for relevance to the article. Consideration was given to all articles with sufficient evidence including systematic review, retrospective studies, and clinical trials.
INTRODUCTION
There will be approximately 165,000 new cases and 30,000 deaths from prostate cancer (PCa) in the United States in 2018.[1] For the localized PCa, treatment options include, but are not limited to radical prostatectomy (RP), external beam therapy, brachytherapy, cryotherapy, or surveillance.[2] Of the patients undergoing curative treatment via RP, approximately 20%–40% may experience a biochemical recurrence (BCR) at some point.[3] Due to the heterogeneous nature of BCR, its management strategies are varied and nuanced and have been further complicated by the advances experienced in the field over the previous two decades. Paradigm shifts in upfront surveillance versus treatment, new recommendations on screening protocols, and technological advances have altered the approaches a modern clinician must take toward BCR management.
The purpose of this review is to provide an up-to-date report on:
The definitions of BCR and their utility in clinical practice
Contributing factors in BCR prevalence
Risk stratification of postprostatectomy patients for BCR
Diagnostic modalities used in confirming and planning treatment for BCR
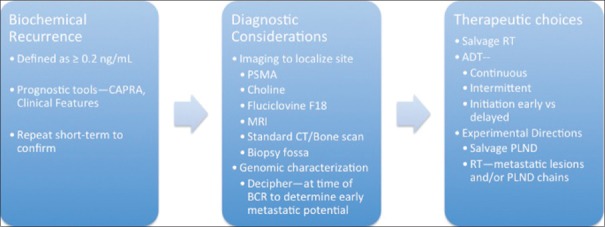
Current strategies for the management of BCR [Figures 1 and 2].
Figure 1.
Biochemical recurrence as 3-part concept
Figure 2.

Biochemical recurrence: High priority research aims
BIOCHEMICAL RECURRENCE DEFINITIONS
Detecting BCR after standard local treatment is important in identifying treatment failure and considering salvage therapy.[4] BCR is diagnosed with posttreatment serum prostate-specific antigen (PSA) levels.[5] The definition of BCR is treatment specific.[6] PSA may remain detectable after radiation therapy as prostatic epithelium remains, whereas, serum PSA levels are expected to be low or undetectable after RP due to the complete removal of prostatic tissue.[7]
Demarcating specific numerical cut-points to define BCR after RP has been a subject of debate in the literature.[8] Definitions vary from any detectable postoperative PSA level to various singular numerical cut-points to consecutive increasing PSA values.[8] The Current American Urological Association and European Association of Urology Guidelines define BCR following RP as an initial PSA value of ≥0.2 ng/mL confirmed by a subsequent PSA value of ≥0.2 ng/mL.[8,9] It is understood that the initial postoperative PSA values should be obtained after 6 weeks to allow for appropriate washout of the residual serum PSA and should be confirmed with a second measurement to rule out a laboratory error.[8] Liu et al. discussed a particular laboratory, in which a positive bias in the laboratory's PSA readings resulted in patients being incorrectly categorized with detectable PSA after prostatectomy when actually their PSA levels were undetectable.[10]
This definition however, has limitations. In some patients, a detectable serum PSA following RP is because of residual benign glands.[11] Also, guideline definitions of BCR do not include supersensitive PSA measurements. One retrospective study demonstrated the usefulness of supersensitive PSA values by correlating a PSA nadir of ≤0.008 versus ≥0.008 (a value only attainable through supersensitive PSA values) with a lower probability of BCR.[12] Furthermore, the utility of BCR is restricted to measuring the outcomes of primary treatment and cannot be used to time the initiation of salvage therapies.[13] Identification of BCR as a standalone benchmark is fairly nonspecific as it does little to detail the origin of biochemical failure and the extent of disease recurrence, though some studies suggest that metastatic relapse is better predicted with a cut-point of ≥0.4 ng/mL.[14] The more widely accepted method of predicting metastatic recurrence includes observing BCR in the context of initial clinical factors (i.e., tumor category, PSA level, PSA velocity, and biopsy Gleason score), pathological findings after surgery, and postfailure PSA kinetics.[15] A retrospective study of 304 men who developed BCR and were not treated with salvage therapy until the development of clinical metastases showed that a PSA doubling time (PSADT) of <10 months, higher prostatectomy Gleason scores (Gleason scores 8–10), and a shorter interval between treatment and PSA failure (≤2 years) were independently associated with distant metastases after PSA failure on multivariate analyses.[4]
Features affecting incidence
Recent changes in the primary treatment options have inevitably impacted the prevalence of BCR in patients after treatment. Both the rise of robotics in performing radical prostatectomies and new guidelines outlining the role of active surveillance as a primary treatment warranted investigation as of how these treatment options affected long-term patient outcomes.
RPs can be performed by an open or a minimally invasive approach, which includes both laparoscopic and robotic techniques. Robot-assisted radical prostatectomies (RARP) were introduced as an alternative surgical treatment for localized PCa in 2000.[16] Since then, the utilization of robotics in prostatectomies has grown significantly over the years, with a usage of over >60% in 2008 and 2009 in the United States as compared to 9.3% in 2003.[17] RARP has been shown to have superior short-term perioperative and postoperative outcomes when compared to open RP (ORP), including lesser blood loss and need for blood transfusions, shorter hospital stay, and fewer positive surgical margins.[17,18,19,20,21,22,23]
The ability of RARP to impact the long-term outcome of BCR, however, is less clear. Liss et al. found a 5-year BCR rate of approximately 14% in patients treated with RARP, with the Gleason grade and pathological stage pT3b or disease involving the seminal vesicles being the strongest predictors for BCR.[24] A case series of men who underwent RARP from a single, high-volume, European center found a BCR rate of 15.2% at a median follow-up of ≥5 years, with BCR being significantly associated with surgeon volumes <100, preoperative PSA >10, pathological stage ≥pT3a, pathological Gleason sum >6, and positive surgical margins on multivariate analysis.[25] A single-surgeon case series of 439 patients who underwent RARP demonstrated that positive surgical margins, pathological Gleason sum ≥8, stage pT3 and higher, and preoperative PSA ≥10 were significantly associated with BCR on multivariate analysis.[26] For reference, one retrospective study of 831 men who underwent ORP by a single surgeon reported a BCR rate of 19% at median follow-up of 52 months.[27]
Evidence on the effect of RARP on BCR in comparison to other surgical techniques is conflicting. In a prospective, longitudinal, observational study comparing 1137 men who underwent ORP to 755 who underwent RARP, Herlemann et al. found no difference in BCR outcomes between the two groups.[28] Ritch et al. and Shapiro et al. found similar oncological outcomes between RARP and ORP in retrospective reviews.[29,30] In a prospective, nonrandomized, observational study with a single surgeon completing his first RARPs, Thompson et al. showed a 35% decreased risk of BCR with RARP when compared to ORP, but only after the surgeon had gained sufficient experience in RARPs.[31] Fujimura et al., in a retrospective observational study in Japan, demonstrated a 40% risk reduction in BCR for patients who underwent RARP as opposed to ORP.[32] Of note, all patients analyzed beyond 2012 were treated with RARP due to policy changes that resulted in coverage of RARP by insurance, and the surgeon experience was not taken into account. A meta-analysis of two systematic reviews and 16 additional studies demonstrated a 59% risk reduction when comparing RARP to laparoscopic RP (LRP).[33] Tewari et al. showed a significantly lower overall positive surgical margin rate for RARP as compared to LRP after propensity adjustment; another prospective cohort study showed a significant association between positive surgical margins and BCR.[34,35] Magheli et al., however, showed higher overall positive surgical margins when comparing RARP to both ORP and LRP after propensity score matching.[36]
The effects of active surveillance and the United States Preventive Services Task Force on biochemical recurrence frequency
The National Comprehensive Cancer Network (NCCN) has recommended active surveillance as the first-line treatment for patients with very low-risk or low-risk PCa and a life expectancy of ≥10 years.[37] Furthermore, in an attempt to curb overtreatment of clinically insignificant PCa, the NCCN guidelines state that men with favorable intermediate-risk PCa (predominant Gleason Grade 3 pathology, positive core biopsy percentage <50%, and ≤1 NCCN intermediate risk factor, e.g., clinical stage pT2b/c or PSA value of 10–20 ng/mL) may also consider active surveillance as the first-line management.[37] While studies analyzing the effect of active surveillance in this patient sub-population on BCR rates are limited, some studies have retrospectively analyzed the outcomes of patients who meet the NCCN's favorable intermediate-risk criteria, but underwent radical prostatectomies in accordance with the standard of care at the time.
Gearman et al. found that men with Gleason Grade 2 disease, who would still be considered as favorable intermediate risk, had a 10-year BCR risk of 18.8% in comparison to 11.1% in the Gleason Grade 1 disease group, implying that men who are offered active surveillance for favorable intermediate-risk disease should be warned of the increased risk of BCR.[38] Other studies confirm that the NCCN's less aggressive surveillance approach may be a reasonable method of achieving similar BCR outcomes for favorable intermediate-risk disease. In a study of 3686 men who underwent RP, Aghazadeh et al. showed that there was no statistically significant difference in time to BCR between low-risk and favorable intermediate-risk groups.[39] Of note, patients with low-risk, favorable intermediate-risk, and unfavorable intermediate-risk (in that they did not meet the NCCN criteria for favorable intermediate-risk) noted a difference 5-year BCR-free survival rates, with rates of 93%, 87%, and 79%, respectively.[39] Beauval et al. studied 2928 intermediate-risk patients who underwent RP and bilateral lymph node dissection at seven academic centers and found that patients with pathologically favorable PCa, defined as low-grade organ-confined disease, had a 94.2% BCR-free survival rate whereas intermediate-risk patients with unfavorable PCa had a BCR-free survival rate of 74.4%.[40] While these studies suggest better BCR outcomes and potential overtreatment of the patients with favorable intermediate-risk disease, a prospective, randomized trial analyzing BCR rates in favorable intermediate-risk groups treated with active surveillance is required to strongly solidify the role of active surveillance in this risk group.
In 2011, the United States Preventive Services Task Force (USPSTF) made recommendations against the PSA-based screening for PCa.[41] This grade “D” recommendation was based on the concerns of the impact of overdiagnosis of otherwise indolent PCa and the potential, physical, and emotional impacts of treating these incidentally found lesions. This recommendation was met with considerable controversy and opposition from the urologic community. Although recent iterations of the USPSTF recommendations have been changed, there was an interest in what effects, if any, these changes may have had on the subsequent screening, treatment, and recurrence patterns. Several studies have noted a decline in PCa diagnosis since the recommendation change, with the largest decrease in the 1st year. These have ranged from 12% to 28% in large studies, but the data alluding to its impact on BCR is more elusive.[41,42,43] While the specific data of how this affects intermediate and high-risk cancer groups may be lacking, inferences can be drawn. Gaylis et al. studied patients referred for elevated PSAs in a community setting from 2011 to 2015. While the overall referral and biopsy rate decreased, there was a significant increase in men presenting with PSAs > 10 (28.1%–36.8%, P = 0.009) as well as patients with Gleason 7 or higher disease (51.6%–69.7%, P = 0.0001).[44] In a larger study evaluating SEER data, there was an increase in the incidence of metastatic PCa from 2009 to 2013, reported as an annual percentage change of 3.1% in all the age groups (P < 0.05).[45] Of particular concern, increase in incidence was reported in men aged 45–64 years, while those aged 75 years and higher were noted to have a significant decline in the incidence (−2.07%, P < 0.05). It remains unclear if these trends will lead to an increase in BCR rates, but the possible public health implications of the changes in USPTF recommendation with a rise in proportion of patients with advanced-stage disease have been made evident and longer follow-up and further studies will be required to elucidate their full effects.
Postprostatectomy risk stratification
The development of simple scoring systems based on clinical parameters is of relevance in evaluating patients who may be susceptible to BCR. One of the most widely used scoring systems is the Cancer of the Prostate Risk Assessment (CAPRA) score. Originally described in 2006, the scoring system is based on common clinical factors including PSA, Gleason score, T stage, patient age, and percentage of biopsy positive cores. With a score range of 0–10, 5-year RFS was noted at 85% for patients with a score of 0–1 and 8% in patients with a score of 7–10.[46] Numerous validation studies have been performed after the initial report. A 2013 systematic review and meta-analysis looked at seven separate validation studies with a total cohort of over 12,000 patients and found that CAPRA was able to accurately predict 3-year biochemical RFS (BRFS) in postprostatectomy patients, but underpredicted the RFS at 5 years.[47] Subsequent variants of the CAPRA score have been developed including those that look at the postsurgical setting (CAPRA-S) and in patients undergoing primary androgen deprivation therapy (ADT) (J-CAPRA).[48] While the use of CAPRA and similar scoring systems is of relevance in predicting patients who are likely to develop BCR, however, its predictive ability once BCR has been established is unclear. A 2016 retrospective study looked at postsurgical patients who had received subsequent EBRT, stratified based on the CAPRA-S risk scores. While there was an observed trend toward CAPRA-S scores predicting survival (P = 0.058), the score itself was only able to accurately predict time to BCR and freedom from palliative hormonal deprivation.[49] This suggests little utility of CAPRA score at this point in patients already diagnosed with BCR.
Recent attention has been turned to the evaluation of application of genomic platforms to risk-stratification systems. A 2016 study evaluated 260 intermediate-to-high-risk patients who had undergone prostatectomy with a Gleason score of 7 or greater and CAPRA-S score of 3 or higher. Ninety-nine patients in this cohort developed metastatic disease and the authors found that the inclusion of the Decipher® assay increased the prognostic performance of the CAPRA-S risk model.[50] Although clearly limited by its retrospective nature, it is clear that a combination of scoring systems with genomic efforts will likely play a larger role in the future evaluation of patients.
Biochemical recurrence in the hypogonadal male
Sex hormone levels have often been associated with mislead beliefs regarding their influence on PCa. Studies have conversely suggested that low hormonal levels may be associated with more advanced disease states. A 2013 study showed that patients with low free testosterone levels were associated with higher tumor stage and lymph node status, while low total testosterone levels were associated with higher Gleason scores.[51] In this study, a serum cutoff point of <0.047 μg/L was associated with these poorer findings. Salonia et al. demonstrated in a cohort of 605 patients that lower total testosterone and sex hormone-binding globulin (SHBG) levels were associated with early BCR.[52] Further studies are required to fully elucidate whether hypogonadism affects survival outcomes in PCa patients; however, there is also tremendous interest from a quality of life standpoint, and in fact, the current studies are addressing the safety of hormonal replacement in the PCa patient. The larger question is how to manage these hypogonadal patients once identified. At present, there are no recommended alternative strategies, but hormonal levels should be taken into consideration along with other risk factors while designing a treatment approach. Further studies are needed to determine if these patients should be managed differently.
DIAGNOSTICS IN BIOCHEMICAL RECURRENCE
PSA values serve as the foundation for initial suspicion and defining of BCR. However, confirmation of BCR with diagnostics including imaging modalities, prostatic bed biopsies, and genomic-based platforms provide key information to confirm recurrent disease and provide better treatment choices for optimal patient outcomes.
Use of novel imaging modalities for biochemical recurrence
The current guidelines recommend considering imaging studies after BCR is detected. Conventional modalities such as transrectal ultrasound (TRUS), CT scan, and technetium-99 m-MDP bone scan are often used, although their utility at low PSA levels has been questioned.[53] Guideline-based recommendations support judicious use, with focus on various factors, including a patient's initial disease risk stratification preintervention, pathologic stage and grade, PSADT, and postintervention PSA to determine imaging timing.[3] Furthermore, a PSA increase itself is not sufficient information, and the distinction must be made between a PSA-only relapse, local recurrence, distant metastatic disease, and a combination of local and distant recurrences.[54] The clinician must also be aware of the data supporting the initiation of salvage therapy at a lower PSA threshold is more efficacious for the patient.[3] With this in mind, novel imaging techniques are attempting to accomplish two major goals; identifying potentially dangerous disease recurrences with a high level of accuracy at the lowest PSA levels possible.
Traditional imaging techniques demonstrate varying level of sensitivities and PSA thresholds for detecting recurrent disease. TRUS techniques may perhaps be the most simplistic and cost-effective measure for identifying recurrences, and have been demonstrated to be more effective than digital rectal examinations, but have clear drawbacks.[55] While being advantageous for specific anatomic locations, particularly the bladder neck and vesicourethral anastomosis, TRUS can identifying a primarily hypoechoic lesion at a PSA >2.0 but <50% of recurrent carcinomas are detectable at a PSA of <0.5.[56]
Bone scan has been shown to be equally ineffective at lower PSA thresholds. Early studies demonstrated the overall probability of a positive bone scan after PSA only recurrence at <5% with PSA values above 40 ng/ml as a threshold for meaningful findings.[57] A later study by Kane et al. demonstrated a 9.4% positive rate in patients with postprostatectomy BCR, with PSA velocity of >0.5 ng/ml/month being predictive of positive findings.[58] An additional study evaluating a cohort of 330 patients with BCR after prostatectomy demonstrated a 14.5% total positive rate for metastatic disease on bone scan, but only 4% positive rate inpatients with a PSA level between 0 and 10 (median value 8.4).[59] PSA velocity was also demonstrated to be predictive of positive findings on multivariate analysis.
Conventional computed tomography (CT) imaging has shown similar findings, with large studies demonstrating an approximate 14% positive rate within 3 years of BCR.[58] In the case of CT, PSA velocity was less effective at predicting positive findings, but when used in a logistic regression model along with CT, it was more effective.[58] However, studies have demonstrated that the mean PSA velocities associated with a positive CT findings often exceeds 30 ng/ml/year, significantly higher than practical for clinical practice.[53,57]
Positron emission tomography (PET) imaging with conventional radiotracers such as fluorodeoxyglucose poses challenges related to urological malignancies, particularly with PCa, primarily due to a low glycolysis rate in PCa as well as isotope collection in the bladder, which makes local recurrences more difficult to identify.[53] With the advent of new radiotracers, PET imaging may be more useful in PCa.
Choline-based PET radiotracers have been extensively evaluated in PCa. Choline serves as a precursor for phosphatidylcholine, a cellular membrane component. Choline accumulation in proliferating tumor cell membranes allows for the detection of radiolabeled choline analogs in PCa patients.[60] The two most commonly used radiotracers include 11C-Choline and 18F-Choline. The applicability of choline-based PET in BCR was evaluated in a meta-analysis, which evaluated 11 studies.[61] They found that the pooled sensitivity was 61% with a specificity of 97%, although a high level of variability was appreciated amongst the studies.[61] This low sensitivity is perhaps one of the most significant disadvantages of choline-based PET. It has also been argued that the detection rate of this modality was suboptimal due to the slow proliferation of cancer cells.[62] Kitajama et al. compared multiparametric magnetic resonance imaging (MRI) with endorectal coil to 11C-choline PET and found that local recurrences were confirmed in only 54.1% of patients undergoing PET.[63] However, it was noted that choline-based PET was much more effective in detecting lymph node metastases with a sensitivity and specificity of 92.3% and 100%, respectively. 11C-choline PET has been unable to demonstrate significant utility at low PSA threshold levels. Castellucci et al. demonstrated the ability to detect recurrence in only 28% of patients with PSA levels of <1.5 and the NCCN guideline data suggest a minimum threshold level of 2.0 ng/mL.[64]
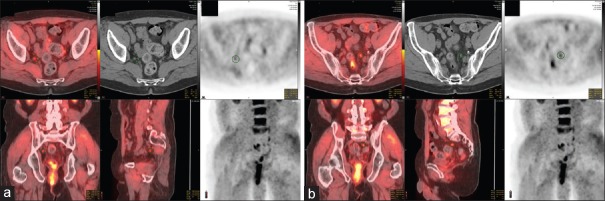
18F-fluciclovine PET/CT is an additional evolving imaging strategy. This synthetic amino acid analog of L-leucine is utilized due to the increased energy demand of the prostate carcinoma. It is taken up preferentially by specialized amino acid transporters, particularly ASCT2. Of particular utility, it does not undergo additional metabolism in the PCa cells, leading to intracellular accumulation.[65] Its half-life is considerably longer than other radiotracers, making it more practical in clinical use.[60] This modality has shown particular promise in relation to imaging in BCR patients. In a 2014 prospective study, 93 patients underwent imaging with 18F-fluciclovine compared to 111In-capromab pendetide (ProstaScint). In this head-to-head study, 18F-fluciclovine was noted to have a 90.2% sensitivity, 40% specificity, and 75.3% positive predictive value in identifying patients with prostatic bed disease. This was superior to the comparison modality which had a 67.2% sensitivity, but a higher specificity of 56.7%.[66] A meta-analysis in 2015 further evaluated the performance of fluciclovine PET. A total of 6 studies with a total cohort of 251 patients were evaluated. Pooled sensitivity across the studies was found to be 87% with a specificity of 66%.[67] PSA threshold values for optimal use of fluciclovine PET are more challenging due to dosing differences between the studies which may limit detection rates.[65] Studies to date suggest the most optimal threshold level for fluciclovine PET imaging to be at 2.0 ng/ml, although there have been some findings to suggest up to a 65% positive rate in patients with PSA levels between 1.0 and 2.0 ng/ml.[62,65,68] [Figure 3a and b] illustrates a challenging case of BCR after RP, requiring salvage pelvic lymph node dissection (sPLND). As noted, highly sensitive and specific imaging can lead to a surgical challenge of retrieving nodes that are active on the imaging but invisible during surgery.
Figure 3.
A 66-year-old presented 3 years out from radical prostatectomy with rising prostate-specific antigen to 1.62. Radical prostatectomy pathology was pT2 Gleason 4 + 3 Nx R0. Standard imaging showed no metastases and fluciclovine positron emission tomography showed bilateral hypogastric lymph nodes as indicated on the circle on the various fused and computed tomography images on the right (a) and left (b). A robot-assisted salvage extended template lymph node dissection was performed with tumor seen in 2/11 nodes seen on the right, but 0/10 on the left. Prostate-specific antigen declined to 0.3 and stable at 15 months follow-up. The case demonstrates multiple challenges in biochemical recurrence: (1) patient selection, (2) ideal imaging modality and localizing disease, (3) performing salvage lymph node dissection without a visible target, and (4) measuring and interpreting clinical benefit
Prostate-specific membrane antigen (PSMA) is a folate hydrolase cell surface glycoprotein, localized to the cytoplasm of PCa cells in benign conditions.[65] As malignant transformation occurs, PSMA is then transferred to the luminal surface of the prostatic ducts.[69] PSMA has been shown to be over-expressed by 100–1000 folds in cancer tissue as compared to the normal tissue.[70] These findings have sparked interest in PSMA as a possible radiotracer target. The most commonly used PSMA inhibitor in PET imaging is gallium-68 PSMA-HBED-CC (Ga-68 PSMA-11).[65] Compared to other modalities, PSMA-PET has shown an ability to detect recurrences at lower PSA thresholds. A 2017 study looked at 1007 patients undergoing PSMA-PET and noted positive lesions in 48% of patients with a PSA <0.5 ng/ml and 73% for values between 0.5 and 1.0 ng/ml.[71] Additional studies have evaluated the utility of PSMA-PET at ultra-low PSA levels and noted detection rates >50% for PSA values between 0.20 and 0.29 ng/ml.[72] Further prospective studies and head-to-head trials are needed to further evaluate the utility of PSMA-PET and compare it with conventional options.
Of particular interest is whether the advent of imaging modalities for BCR detection at a lower threshold has impacted treatment management. Several studies have supported this. Afaq et al. performed a retrospective evaluation of 100 patients with BCR who underwent PSMA-PET evaluation and found that, after imaging, management strategies changed in 39% of patients, including 33.8% of patients whose initial treatment was RP.[73] In a smaller study looking at a cohort of 40 patients who underwent RP, it was found that the therapeutic approach was changed in 70% of these patients after PSMA-PET. It should be noted that, in addition to patients who had BCR after RP, the study also included patients post salvage RT and ADT. Nevertheless, these studies demonstrate the potentially profound impact of PSMA-PET on management of PCa. The current NCCN guideline statements support the use of ADT or salvage RT in the PET-positive patient.
The use of MRI in the detection of BCR, particularly the use of multiparametric MRI, has been established. Among functional sequences, studies suggest that dynamic contrast-enhanced (DCE) modalities may be most effective at detecting BCR. Roy et al. evaluated a cohort of BCR patients with DCE, diffusion-weighted imaging (DWI), and 3D (1)H-MR spectroscopy (MRS) techniques. They divided their cohort into those patients who were postprostatectomy as well as postexternal beam therapy. In postprostatectomy group, the sensitivities for DCE, DWI, and MRS techniques were 100%, 71%, and 57%, respectively.[74] Further attention has focused on the combination of DCE and DWI modalities as a way to increase the overall detection rate of recurrences. From large systematic reviews, MRS with DCE has shown the highest pooled sensitivities at 89%, followed by DWI with T2 imaging (82%) and DCE with T2 imaging (82%).[75] Of these combinations, the highest specificity was noted in the DCE with T2 imaging group (92%). These studies suggest that combination imaging is likely the modality that will be most effective in detecting recurrences but further investigation is required to better define their role.
Utility of prostatic bed biopsies to detect recurrences
Traditional TRUS-guided biopsy techniques have been employed to detect local recurrences at the prostate bed after BCR. However, these have limited detection rates based on varying PSA levels. In an early study, Saleem et al. evaluated 91 postprostatectomy patients with an abnormal DRE or PSA >0.2 ng/ml and found that, for those patients with PSA values <1, 24% had a positive biopsy.[76] Looking at a PSA threshold of 0.5 ng/ml or less in patients with a negative DRE, no positive biopsies were identified. A 2005 study evaluated 100 postprostatectomy patients for prostatic fossa biopsy. They found a sensitivity for TRUS to detect recurrence at 86.2%, but with a low specificity of 53.5%. They also noted that none of the patients with a PSA level of <0.5 and a negative DRE had positive biopsy findings.[77]
Linder et al. evaluated the utility of MRI in identifying early prostatic fossa recurrence before TRUS biopsy. One hundred and eighty-seven patients were identified who had endorectal coil MRI followed by TRUS biopsy. Lesions were identified in 94% of patients on MRI with a median PSA of 0.59 ng/ml.[78] Importantly, they noted that when a lesion was identified on MRI, the positive biopsy rate was 65%. Lesion size on imaging was a significant predictor of positive biopsy, with a rate of 51% with a lesion <1 cm, but 88% on lesions >2 cm.
The applicability of recurrence detection by novel imaging techniques applied to prostatic bed biopsies has been assessed in several studies. Recently, Cha et al. evaluated a cohort of 43 patients with postprostatectomy BCR and a median PSA level of 0.71 via several modalities including T2 weighted imaging, DWI, and DCE. Following imaging, all patients underwent TRUS-guided biopsy of the prostatic bed. They found that combined imaging modalities were more accurate at predicting local recurrence than T2-weighted imaging alone.[79]
Genomic-based platforms in biochemical recurrence
Large variability in clinical course of PCa patients, as well as the numerous different treatment options available, has ushered in the era of personalized medicine. Numerous genomic-based markers are currently available to patients at all points in the cancer diagnosis/care timeline. These provide information on the potential for aggressive disease, whether treatment or active surveillance may be more beneficial after a diagnosis, as well as determining the likelihood of BCR after PCa intervention. There has been a surge in discovery studies evaluating the prognostic value of gene expression panels for clinical endpoints. To this end, various studies have evaluated postprostatectomy BCR risk after prostatectomy with positive results that have led to the development of new scoring systems and nomograms that have outperformed previous models.[80] Cell cycle progression (CCP) risk scores have been developed as an example. These models are based on the concept of increased CCP activity translating to higher cell activity and therefore malignant risk. Validation studies have demonstrated increasing hazard ratios for disease recurrence based on progressively increasing CCP scores.[81] The challenge with genomic-based approaches is due to the inherent variability in the natural history of disease among patients. Due to this complexity, it is unlikely that any single genetic abnormality will provide enough prognostic information to be of clinical utility, but more likely that a combination will be necessary.[80]
Decipher® (GenomeDx Biosciences) is the most widely available commercial assay with relevance to BCR available today. This 22-gene expression assay is applicable to men treated with RP to predict the 5-year risk of metastatic PCa. This allows physicians to distinguish between which patients are likely to be restricted to long-term BCR as opposed to early metastatic relapse of disease, with the latter requiring more aggressive postoperative treatment regimens.[4] A genomic classifier score based on the microarray on formalin-fixed paraffin embedded tissue is generated, and patients are assigned as high, average, or low risk of metastatic disease.[82] Large tertiary center databases were used in the initial evaluation of these genomic classifier scores. A 2013 study from the Mayo Clinic evaluated 639 patients who underwent RP over a 14-year period. The genomic classifier was able to achieve a receiver operating characteristic curve of 0.75, which outperformed common prognostic factors such as Gleason score.[83] Additional work from the Mayo Clinic analyzed high-risk patients including those with a PSA >20 ng/ml, Gleason score 8 or greater, pT3b disease, or a Mayo Clinic nomogram score of 10 or greater.[84] The genomic classifier score was found to be a primary predictor of metastatic disease in this high-risk cohort, with a 5-year rate of 2.4%, 6.0%, and 22.5% in patients with low-, intermediate-, and high-risk scores, respectively.[84]
A 2015 study from the Cleveland Clinic also validated Decipher® in a cohort of 169 patients with high-risk disease with pathological node-negative disease, undetectable post-RP PSA, and no adjuvant therapy as a means to evaluate patients at risk for rapid metastasis (within 5 years). Their rapid metastasis patients developed recurrent disease at a median of 2.3 years and Decipher® was found to be a significant predictor as well as carried a higher prognostic value than the other scoring systems studied.[85] The current NCCN guidelines support the use of the Decipher® assay for those patients with PSA persistence/recurrence after prostatectomy as a category 2B recommendation.[3]
SALVAGE THERAPIES AFTER BIOCHEMICAL RECURRENCE
Several treatment options exist for patients with BCR after prostatectomy. The current guidelines discuss the use of salvage radiotherapy and ADT. sPLND in the event of nodal recurrence is a promising treatment option that has yet to be officially recommended by the current guidelines.
Salvage radiotherapy after biochemical recurrence
A portion of patients without high-risk features, or those who elect for observation postprostatectomy, will experience BCR and will be offered salvage radiotherapy. Several studies have demonstrated that salvage therapy reduces distant metastatic disease development and improves PCa specific mortality.[86] Evidence suggests that pretreatment PSA correlates highly with outcomes in patients undergoing salvage therapy. A 2014 meta-analysis evaluating ten retrospective studies looked at BRFS based on early- or late-salvage radiation therapy. They found that 5-year BRFS was 71.1% after early-salvage radiotherapy (PSA <0.5 ng/ml).[87] This was compared to data, suggesting that the BRFS rate for those treated with salvage therapy at PSA >0.5 ng/ml is as low as 46%.[88] However, these studies did little to address how BRFS affected clinical outcomes in these patient cohorts.
Stish et al. evaluated a cohort of 1106 patients receiving salvage radiotherapy over a 16-year period with a median follow-up of 8.9 years. The median time of RP to salvage therapy was 2.8 years with a median dose of 68 Gy. The authors used a PSA threshold of <0.5 ng/ml compared to >0.5 ng/ml and found that those treated with early-salvage therapy had a significantly lower risk of distant metastatic disease, cause-specific mortality, and overall survival at both 5- and 10-year endpoints.[89]
Treating all the patients with BCR after RP with salvage radiotherapy will result in unnecessary morbidity and overtreatment.[90,91,92] Ross et al. utilized Decipher to help guide the decision to use salvage radiotherapy in patients with BCR. Fewer than 10% of patients categorized in the low-risk group with Decipher <0.4 developed metastasis within 3 years of BCR, while 73% of men with Decipher ≥0.4 developed metastasis, with 40% within 3 years of BCR.[93] Furthermore, Den et al. demonstrated that for patients with Decipher scores <0.4, there were no reductions in cumulative incidence of metastasis when comparing adjuvant radiotherapy to salvage radiotherapy. For patients with Decipher scores ≥0.4, cumulative 5-year incidence of metastasis was 6% for patients treated with adjuvant radiotherapy group in comparison to 23% for the salvage radiotherapy group, indicated the superiority of adjuvant radiotherapy in patients with higher Decipher scores.[94]
There are limited data demonstrating the efficacy of targeting radiotherapy to metastatic lesions in men with metastatic BCR after RP. Kahn et al. attempted systemic radioimmunotherapy in eight patients who have recurrent disseminated PCa following a failed RP. None of the patients demonstrated response by PSA criteria, and six out of eight patients experienced significant bone marrow toxicity.[95]
The use of androgen deprivation therapy in biochemical recurrence
ADT is an additional option for patients with BCR without evidence of distant metastatic disease, although the current guidelines still caution against its broad use. The decision to proceed with ADT should be based on a thorough discussion with the patient regarding short- and long-term side effects while also considering patient comorbidities and desires.[3] Randomized trial data suggest that early initiation of ADT is more beneficial to patients versus delayed initiation. This landmark study by Duchesne et al. randomized patients who had failed curative therapy or were deemed unsuitable for curative therapy into early versus delayed ADT initiation. Immediate versus delayed time points were defined as within 8 months of randomization versus 2 years.[96] The study randomized 142 and 15 to the immediate and delayed arms, respectively. At 5 years, the overall survival was 86.4% in the delayed arm versus 91.2% in the immediate arm (P = 0.05). While these results support early initiation of ADT, the risk of side effects remains high (36% of patients in the Duchesne study had adverse events requiring hospitalization), and for this reason, guideline consensus recommends considering other factors including PSA velocity and doubling time when selecting patients for ADT. Additional consideration could be given to intermittent ADT based on a large Canadian trial demonstrating noninferiority of intermittent ADT compared to continuous therapy with improved side effect profiles in the intermittent group.[97]
Salvage pelvic lymph node dissection after biochemical recurrence
When BCR appears in the form of positive lymph nodes, sPLND is a treatment option. Considering that the patients with nodal-only recurrence have more favorable outcomes than those with visceral or bone metastasis,[98] the fact that ADT does not yet represent a curative treatment,[99] and the significant toxicity associated with ADT,[100] sPLND may serve as a reasonable alternative to ADT.
Rigatti et al. demonstrated, in a prospective analysis of 72 patients, that 35% with BCR after RP treated with sRLNP were clinical recurrence free at 5-year follow-up.[101] Jilg et al. demonstrated that 42.6% of 52 sPLND patients were free of clinical symptoms for 3 years.[102] Despite these results, there is a lack of long-term data demonstrating the oncological safety of sPLND, limiting its clinical implementation.[103] International guidelines currently recommend sPLND for treatment of BCR after prostatectomy.
CONCLUSION
PCa management is perhaps more challenging for the modern clinician than in the past decades. The advent of new technological advances as well as shifting opinions on disease management has critical implications on care of the day-to-day patient. However, despite this complexity, it is evident that these changes are continually adding vital information to the wealth of knowledge on the subject and bringing clinicians closer to being able to optimize management for all patients, giving them the best chance at long-term control and cure.
Take home messages
Definition of biochemical recurrence – The most common definition is >0.2ng/mL. Useful prognostic information includes preoperative PSA, postoperative Gleason score 8–10, pathologic stage pT3a or higher, positive lymph nodes, and PSA kinetics such as doubling time <10 months and/or <2 years from treatment. Predictors of progression of BCR to metastasis need to be better defined in future.
Imaging – Traditional choline PET is useful if PSA >2 ng/mL. PSMA-PET may be useful at PSA = 0.5ng/mL. PET may be better at lymph node detection, while mp-MRI is used to detect local prostatic bed recurrences. There is a limited role for prostatic bed biopsies. Future needs include identification of the best PET isotope and correlation of its ability to detect relapse early with meaningful treatment changes.
Treatment – Genomic profiling such as Decipher may be useful in patients with RP with high-risk features to determine the role for adjuvant radiotherapy. ADT is an option with risks and benefits. ADT is better given earlier than delayed but consider PSA kinetics. Intermittent ADT may be better than delayed. Salvage PLND can be considered for BCR with positive lymph nodes; however, there is a lack of long-term oncologic benefit. Future needs include a greater understanding of the role of salvage PLND versus earlier interventions with novel systemic therapies.
Financial support and sponsorship:
Nil.
Conflicts of interest:
Dr. Davis has funded research with GenomeDx and their Decipher product which is discussed in this review.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick J. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Insur Med. 1998;30:204–5. [PubMed] [Google Scholar]
- 3.Carroll PR, Parsons JK, Andriole G, Bahnson RR, Barocas DA, Castle EP, et al. NCCN clinical practice guidelines prostate cancer early detection, version 2.2015. J Natl Compr Canc Netw. 2015;13:1534–61. doi: 10.6004/jnccn.2015.0181. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol. 1994;152:1821–5. doi: 10.1016/s0022-5347(17)32394-7. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen ME, Makarov DV, Humphreys E, Mangold L, Partin AW, Walsh PC, et al. Is it possible to compare PSA recurrence-free survival after surgery and radiotherapy using revised ASTRO criterion--“nadir+2”? Urology. 2008;72:389–93. doi: 10.1016/j.urology.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–42. [PubMed] [Google Scholar]
- 8.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The american urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 9.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Tan CH, Badrick T, Loh TP. Moving sum of number of positive patient result as a quality control tool. Clin Chem Lab Med. 2017;55:1709–14. doi: 10.1515/cclm-2016-0950. [DOI] [PubMed] [Google Scholar]
- 11.Furusato B, Rosner IL, Osborn D, Ali A, Srivastava S, Davis CJ, et al. Do patients with low volume prostate cancer have prostate specific antigen recurrence following radical prostatectomy? J Clin Pathol. 2008;61:1038–40. doi: 10.1136/jcp.2008.057794. [DOI] [PubMed] [Google Scholar]
- 12.Furubayashi N, Negishi T, Kashiwagi E, Hirata Y, Taguchi K, Hasegawa Y, et al. Usefulness of ultra-sensitive prostate-specific antigen following radical prostatectomy. Mol Clin Oncol. 2014;2:851–7. doi: 10.3892/mco.2014.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2008;179:S53–9. doi: 10.1016/j.juro.2008.03.138. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Jr, Lilja H. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 15.Lee AK, D’Amico AV. Utility of prostate-specific antigen kinetics in addition to clinical factors in the selection of patients for salvage local therapy. J Clin Oncol. 2005;23:8192–7. doi: 10.1200/JCO.2005.03.0007. [DOI] [PubMed] [Google Scholar]
- 16.Barbash GI, Glied SA. New technology and health care costs – The case of robot-assisted surgery. N Engl J Med. 2010;363:701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 17.Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive vs. open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 18.Alemozaffar M, Sanda M, Yecies D, Mucci LA, Stampfer MJ, Kenfield SA, et al. Benchmarks for operative outcomes of robotic and open radical prostatectomy: Results from the health professionals follow-up study. Eur Urol. 2015;67:432–8. doi: 10.1016/j.eururo.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandaglia G, Sammon JD, Chang SL, Choueiri TK, Hu JC, Karakiewicz PI, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol. 2014;32:1419–26. doi: 10.1200/JCO.2013.53.5096. [DOI] [PubMed] [Google Scholar]
- 20.Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol. 2012;62:431–52. doi: 10.1016/j.eururo.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Tewari A, Srivasatava A, Menon M Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 22.Trinh QD, Sammon J, Sun M, Ravi P, Ghani KR, Bianchi M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: Results from the nationwide inpatient sample. Eur Urol. 2012;61:679–85. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Leow JJ, Chang SL, Meyer CP, Wang Y, Hanske J, Sammon JD, et al. Robot-assisted versus open radical prostatectomy: A contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70:837–45. doi: 10.1016/j.eururo.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 24.Liss MA, Lusch A, Morales B, Beheshti N, Skarecky D, Narula N, et al. Robot-assisted radical prostatectomy: 5-year oncological and biochemical outcomes. J Urol. 2012;188:2205–10. doi: 10.1016/j.juro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Sooriakumaran P, Haendler L, Nyberg T, Gronberg H, Nilsson A, Carlsson S, et al. Biochemical recurrence after robot-assisted radical prostatectomy in a European single-centre cohort with a minimum follow-up time of 5 years. Eur Urol. 2012;62:768–74. doi: 10.1016/j.eururo.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto R, Fashola Y, Scotland KB, Calvaresi AE, Gomella LG, Trabulsi EJ, et al. Risk factors for biochemical recurrence after robotic assisted radical prostatectomy: A single surgeon experience. BMC Urol. 2015;15:27. doi: 10.1186/s12894-015-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antebi E, Eldefrawy A, Katkoori D, Soloway CT, Manoharan M, Soloway MS, et al. Oncological and functional outcomes following open radical prostatectomy: How patients may achieve the “Trifecta”? Int Braz J Urol. 2011;37:320–7. doi: 10.1590/s1677-55382011000300005. [DOI] [PubMed] [Google Scholar]
- 28.Herlemann A, Cowan JE, Carroll PR, Cooperberg MR. Community-based outcomes of open versus robot-assisted radical prostatectomy. Eur Urol. 2018;73:215–23. doi: 10.1016/j.eururo.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Ritch CR, You C, May AT, Herrell SD, Clark PE, Penson DF, et al. Biochemical recurrence-free survival after robotic-assisted laparoscopic vs open radical prostatectomy for intermediate- and high-risk prostate cancer. Urology. 2014;83:1309–15. doi: 10.1016/j.urology.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro EY, Scarberry K, Patel T, Bergman A, Ahn JJ, Sahi N, et al. Comparison of robot-assisted and open retropubic radical prostatectomy for risk of biochemical progression in men with positive surgical margins. J Endourol. 2014;28:208–13. doi: 10.1089/end.2013.0393. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JE, Egger S, Böhm M, Siriwardana AR, Haynes AM, Matthews J, et al. Superior biochemical recurrence and long-term quality-of-life outcomes are achievable with robotic radical prostatectomy after a long learning curve-updated analysis of a prospective single-surgeon cohort of 2206 consecutive cases. Eur Urol. 2018;73:664–71. doi: 10.1016/j.eururo.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Fujimura T, Fukuhara H, Taguchi S, Yamada Y, Sugihara T, Nakagawa T, et al. Robot-assisted radical prostatectomy significantly reduced biochemical recurrence compared to retro pubic radical prostatectomy. BMC Cancer. 2017;17:454. doi: 10.1186/s12885-017-3439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Seo HJ, Lee NR, Son SK, Kim DK, Rha KH, et al. Robot-assisted radical prostatectomy has lower biochemical recurrence than laparoscopic radical prostatectomy: Systematic review and meta-analysis. Investig Clin Urol. 2017;58:152–63. doi: 10.4111/icu.2017.58.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: A systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–5. doi: 10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Pfitzenmaier J, Pahernik S, Tremmel T, Haferkamp A, Buse S, Hohenfellner M, et al. Positive surgical margins after radical prostatectomy: Do they have an impact on biochemical or clinical progression? BJU Int. 2008;102:1413–8. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 36.Magheli A, Gonzalgo ML, Su LM, Guzzo TJ, Netto G, Humphreys EB, et al. Impact of surgical technique (open vs laparoscopic vs robotic-assisted) on pathological and biochemical outcomes following radical prostatectomy: An analysis using propensity score matching. BJU Int. 2011;107:1956–62. doi: 10.1111/j.1464-410X.2010.09795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: Prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 38.Gearman DJ, Morlacco A, Cheville JC, Rangel LJ, Karnes RJ. Comparison of pathological and oncologic outcomes of favorable risk gleason score 3+4 and low risk gleason score 6 prostate cancer: Considerations for active surveillance. J Urol. 2018;199:1188–95. doi: 10.1016/j.juro.2017.11.116. [DOI] [PubMed] [Google Scholar]
- 39.Aghazadeh MA, Frankel J, Belanger M, McLaughlin T, Tortora J, Staff I, et al. National Comprehensive Cancer Network® favorable intermediate risk prostate cancer-is active surveillance appropriate? J Urol. 2018;199:1196–201. doi: 10.1016/j.juro.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 40.Beauval JB, Cabarrou B, Gandaglia G, Patard PM, Ouzzane A, de la Taille A, et al. External validation of a nomogram for identification of pathologically favorable disease in intermediate risk prostate cancer patients. Prostate. 2017;77:928–33. doi: 10.1002/pros.23348. [DOI] [PubMed] [Google Scholar]
- 41.Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 42.Barocas DA, Mallin K, Graves AJ, Penson DF, Palis B, Winchester DP, et al. Effect of the USPSTF grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194:1587–93. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 43.Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–61. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 44.Gaylis FD, Choi JE, Hamilton Z, Dato P, Cohen E, Calabrese R, et al. Change in prostate cancer presentation coinciding with USPSTF screening recommendations at a community-based urology practice. Urol Oncol. 2017;35:663. doi: 10.1016/j.urolonc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 45.Dalela D, Sun M, Diaz M, Karabon P, Seisen T, Trinh QD, et al. Contemporary trends in the incidence of metastatic prostate cancer among US men: Results from nationwide analyses. Eur Urol Focus. 2017 doi: 10.1016/j.euf.2017.04.012. pii: S2405-4569 (17) 30118-9. [DOI] [PubMed] [Google Scholar]
- 46.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The university of california, san francisco cancer of the prostate risk assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meurs P, Galvin R, Fanning DM, Fahey T. Prognostic value of the CAPRA clinical prediction rule: A systematic review and meta-analysis. BJU Int. 2013;111:427–36. doi: 10.1111/j.1464-410X.2012.11400.x. [DOI] [PubMed] [Google Scholar]
- 48.Brajtbord JS, Leapman MS, Cooperberg MR. The CAPRA score at 10 years: Contemporary perspectives and analysis of supporting studies. Eur Urol. 2017;71:705–9. doi: 10.1016/j.eururo.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann M, Delouya G, Alenizi AM, Rajih E, Zorn KC, Taussky D, et al. CAPRA-S predicts outcome for adjuvant and salvage external beam radiotherapy after radical prostatectomy. Can Urol Assoc J. 2016;10:132–6. doi: 10.5489/cuaj.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross AE, Johnson MH, Yousefi K, Davicioni E, Netto GJ, Marchionni L, et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur Urol. 2016;69:157–65. doi: 10.1016/j.eururo.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 51.Schnoeller T, Jentzmik F, Rinnab L, Cronauer MV, Damjanoski I, Zengerling F, et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J Urol. 2013;31:253–9. doi: 10.1007/s00345-012-0902-5. [DOI] [PubMed] [Google Scholar]
- 52.Salonia A, Abdollah F, Capitanio U, Gallina A, Suardi N, Briganti A, et al. Preoperative sex steroids are significant predictors of early biochemical recurrence after radical prostatectomy. World J Urol. 2013;31:275–80. doi: 10.1007/s00345-012-0856-7. [DOI] [PubMed] [Google Scholar]
- 53.Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010;22:46–55. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Martino P, Scattoni V, Galosi AB, Consonni P, Trombetta C, Palazzo S, et al. Role of imaging and biopsy to assess local recurrence after definitive treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU) World J Urol. 2011;29:595–605. doi: 10.1007/s00345-011-0687-y. [DOI] [PubMed] [Google Scholar]
- 55.Kapoor DA, Wasserman NF, Zhang G, Reddy PK. Value of transrectal ultrasound in identifying local disease after radical prostatectomy. Urology. 1993;41:594–7. doi: 10.1016/0090-4295(93)90114-p. [DOI] [PubMed] [Google Scholar]
- 56.Scattoni V, Roscigno M, Raber M, Montorsi F, Da Pozzo L, Guazzoni G, et al. Multiple vesico-urethral biopsies following radical prostatectomy: The predictive roles of TRUS, DRE, PSA and the pathological stage. Eur Urol. 2003;44:407–14. doi: 10.1016/s0302-2838(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 57.Cher ML, Bianco FJ, Jr, Lam JS, Davis LP, Grignon DJ, Sakr WA. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998;160:1387–91. [PubMed] [Google Scholar]
- 58.Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–11. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 59.Dotan ZA, Bianco FJ, Jr, Rabbani F, Eastham JA, Fearn P, Scher HI. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol. 2005;23:1962–8. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li R, Ravizzini GC, Gorin MA, Maurer T, Eiber M, Cooperberg MR, et al. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:4–21. doi: 10.1038/s41391-017-0007-8. [DOI] [PubMed] [Google Scholar]
- 61.Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, et al. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43:55–69. doi: 10.1007/s00259-015-3202-7. [DOI] [PubMed] [Google Scholar]
- 62.Nanni C, Schiavina R, Rubello D, Ambrosini V, Brunocilla E, Martorana G, et al. The detection of disease relapse after radical treatment for prostate cancer: Is anti-3-18F-FACBC PET/CT a promising option? Nucl Med Commun. 2013;34:831–3. doi: 10.1097/MNM.0b013e3283636eaf. [DOI] [PubMed] [Google Scholar]
- 63.Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, et al. Detection of recurrent prostate cancer after radical prostatectomy: Comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med. 2014;55:223–32. doi: 10.2967/jnumed.113.123018. [DOI] [PubMed] [Google Scholar]
- 64.Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase<1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38:55–63. doi: 10.1007/s00259-010-1604-0. [DOI] [PubMed] [Google Scholar]
- 65.Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F] FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J Urol. 2014;191:1446–53. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: A meta-analysis. Acta Radiol. 2016;57:487–93. doi: 10.1177/0284185115581541. [DOI] [PubMed] [Google Scholar]
- 68.Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, et al. Recurrent prostate cancer detection with anti-3-[(18)F] FACBC PET/CT: Comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773–83. doi: 10.1007/s00259-016-3383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 70.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 71.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–9. doi: 10.1111/bju.13397. [DOI] [PubMed] [Google Scholar]
- 73.Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. doi: 10.2967/jnumed.117.192625. [DOI] [PubMed] [Google Scholar]
- 74.Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013;200:W361–8. doi: 10.2214/AJR.12.9106. [DOI] [PubMed] [Google Scholar]
- 75.Sandgren K, Westerlinck P, Jonsson JH, Blomqvist L, Thellenberg Karlsson C, Nyholm T, et al. Imaging for the detection of locoregional recurrences in biochemical progression after radical prostatectomy – A systematic review. Eur Urol Focus. 2017 doi: 10.1016/j.euf.2017.11.001. pii: S2405-4569 (17) 30257-2. [DOI] [PubMed] [Google Scholar]
- 76.Saleem MD, Sanders H, Abu El Naser M, El-Galley R. Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology. 1998;51:283–6. doi: 10.1016/s0090-4295(97)00509-8. [DOI] [PubMed] [Google Scholar]
- 77.Naya Y, Okihara K, Evans RB, Babaian RJ. Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology. 2005;66:350–5. doi: 10.1016/j.urology.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Linder BJ, Kawashima A, Woodrum DA, Tollefson MK, Karnes J, Davis BJ, et al. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol. 2014;21:7283–9. [PubMed] [Google Scholar]
- 79.Cha D, Kim CK, Park SY, Park JJ, Park BK. Evaluation of suspected soft tissue lesion in the prostate bed after radical prostatectomy using 3T multiparametric magnetic resonance imaging. Magn Reson Imaging. 2015;33:407–12. doi: 10.1016/j.mri.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Boström PJ, Bjartell AS, Catto JW, Eggener SE, Lilja H, Loeb S, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033–44. doi: 10.1016/j.eururo.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Cooperberg MR, Simko JP, Cowan JE, Reid JE, Djalilvand A, Bhatnagar S, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31:1428–34. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 82.Marrone M, Potosky AL, Penson D, Freedman AN. A 22 gene-expression assay, decipher® (GenomeDx biosciences) to predict five-year risk of metastatic prostate cancer in men treated with radical prostatectomy. PLoS Curr. 2015;7 doi: 10.1371/currents.eogt.761b81608129ed61b0b48d42c04f92a4. pii: ecurrents.eogt.761b81608129ed61b0b48d42c04f92a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One. 2013;8:e66855. doi: 10.1371/journal.pone.0066855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karnes RJ, Bergstralh EJ, Davicioni E, Ghadessi M, Buerki C, Mitra AP, et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol. 2013;190:2047–53. doi: 10.1016/j.juro.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klein EA, Yousefi K, Haddad Z, Choeurng V, Buerki C, Stephenson AJ, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur Urol. 2015;67:778–86. doi: 10.1016/j.eururo.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 86.Isharwal S, Stephenson AJ. Post-prostatectomy radiation therapy for locally recurrent prostate cancer. Expert Rev Anticancer Ther. 2017;17:1003–12. doi: 10.1080/14737140.2017.1378575. [DOI] [PubMed] [Google Scholar]
- 87.Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65:1034–43. doi: 10.1016/j.eururo.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 88.Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316–22. doi: 10.1016/j.ijrobp.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 89.Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;34:3864–71. doi: 10.1200/JCO.2016.68.3425. [DOI] [PubMed] [Google Scholar]
- 90.Patel AR, Stephenson AJ. Radiation therapy for prostate cancer after prostatectomy: Adjuvant or salvage? Nat Rev Urol. 2011;8:385–92. doi: 10.1038/nrurol.2011.80. [DOI] [PubMed] [Google Scholar]
- 91.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013;189:S34–42. doi: 10.1016/j.juro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 92.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012;109:32–9. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ross AE, Feng FY, Ghadessi M, Erho N, Crisan A, Buerki C, et al. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis. 2014;17:64–9. doi: 10.1038/pcan.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Den RB, Yousefi K, Trabulsi EJ, Abdollah F, Choeurng V, Feng FY, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol. 2015;33:944–51. doi: 10.1200/JCO.2014.59.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD, et al. Aphase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14:99–111. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 96.Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D’Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–37. doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 97.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ, et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 99.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–11. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Does comorbidity influence the risk of myocardial infarction or diabetes during androgen-deprivation therapy for prostate cancer? Eur Urol. 2013;64:159–66. doi: 10.1016/j.eururo.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rigatti P, Suardi N, Briganti A, Da Pozzo LF, Tutolo M, Villa L, et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C] choline positron emission tomography/computed tomography. Eur Urol. 2011;60:935–43. doi: 10.1016/j.eururo.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 102.Jilg CA, Rischke HC, Reske SN, Henne K, Grosu AL, Weber W, et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol. 2012;188:2190–7. doi: 10.1016/j.juro.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 103.Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: Results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015;67:299–309. doi: 10.1016/j.eururo.2014.02.011. [DOI] [PubMed] [Google Scholar]