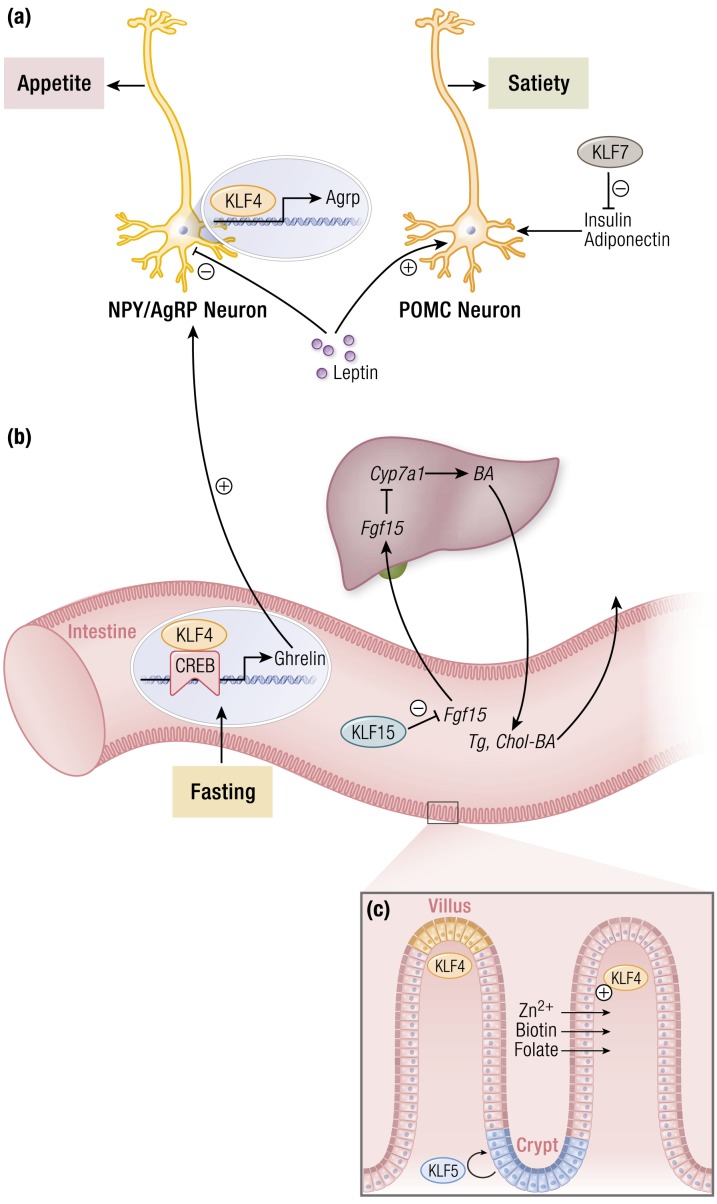
Figure 1.
KLF regulation of nutrient acquisition and absorption. (a) Multiple KLFs regulate appetite and feeding behavior centrally and peripherally. Within the hypothalamus, KLF4 expression promotes expression of AgRP to stimulate appetite. Anorexigenic hormones such as leptin inhibit neuronal KLF4 expression, thereby reducing appetite. Peripherally, KLF4 is induced by fasting and cooperates with CREB to augment ghrelin production. This, in turn, propagates orexigenic signaling in the brain. In addition, KLF7 inhibits production of key satiety-mediating factors that would otherwise ultimately signal through POMC neurons to reduce feeding. (b) Intestinal KLF15 promotes hepatic bile acid production through a feedback loop involving inhibition of FGF15 production within the intestines, ultimately relieving FGF15-mediated inhibition of CYP7A1. This reduces intestinal absorption of triglycerides and cholesterol. (c) Intestinal KLFs regulate proper barrier function and absorptive capacity. KLF4 increases specific nutrient transporters to maintain a pool of important cofactors for many metabolic processes. These include zinc, biotin, and folate transporters. Intestinal barrier function is preserved through a coordination of intestinal stem cell self-renewal and eventual differentiation. KLF5 expression in the crypts maintains available populations of intestinal stem cells. Conversely, KLF4 expression is highest at the villi tips and is associated with intestinal cell differentiation. Abbreviations: AgRP, Agouti-related peptide; BA, bile acid; CREB, cAMP response element binding protein; CYP7A1, cytochrome P450 family 7 subfamily A member 1; FGF15, fibroblast growth factor 15; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; TG, triglyceride; chol, cholesterol. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]