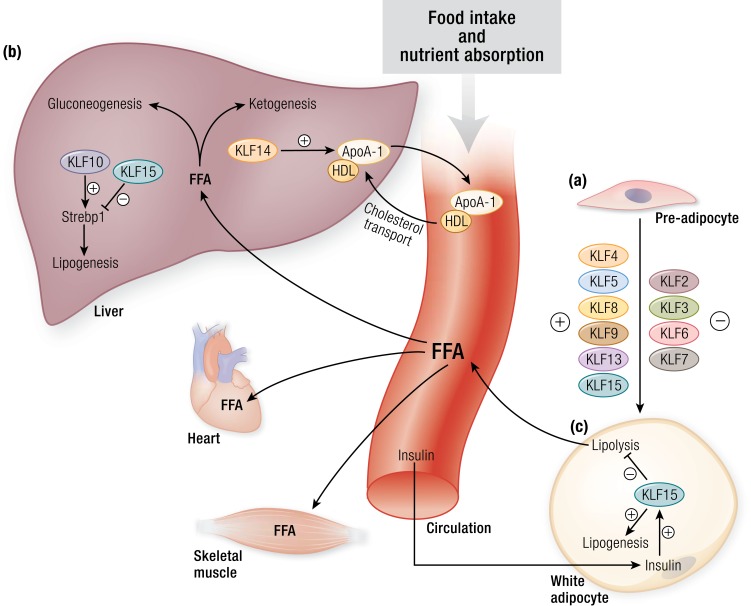
Figure 3.
KLFs in adipogenesis, lipid partitioning, and lipid uptake. (a) KLF4, KLF5, KLF8, KLF9, KLF13, and KLF15 act as positive regulators of adipocyte development, whereas, KLF2, KLF3, KLF6, and KLF7 act as negative regulators. Several of these KLFs interact with nuclear factors such as the PPARs and C/EBPs and coregulators such as CtBP to control adipogenesis. Inactivation of KLFs result in impaired WAT differentiation and development. (b) KLFs play an important role in hepatic lipid metabolism. KLF14 is a transcriptional regulator of Apoa1 and consequently affects systemic HDL levels and cholesterol efflux. Hepatic KLF10 and KLF15 regulate transcription of Srebp1. These transcription factors affect several genes involved in lipogenesis and in gluconeogenesis. (c) In the fed state, insulin induces WAT KLF15 expression, which consequently drives lipogenesis and inhibits lipolysis. Conversely, loss of WAT KLF15 leads to increased lipolysis, thus contributing to increased FFAs in the circulation. These FFAs are then taken up and used by different organs such as the heart and skeletal muscle. ApoA1, apolipoprotein A1; CtBP, C-terminal binding protein 1; FFA, free fatty acid; HDL, high-density lipoprotein; Srebp1, sterol regulatory element-binding protein 1. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]