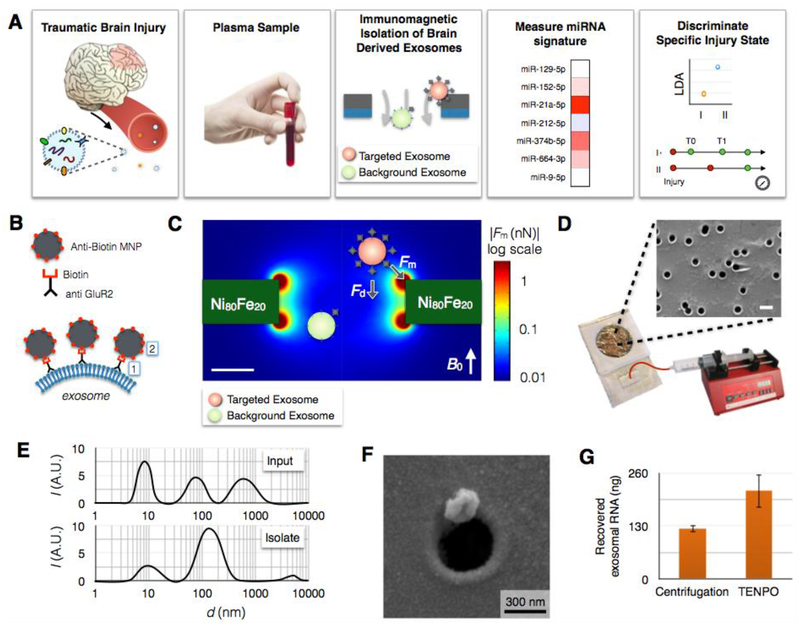
Fig. 1. Immunomagnetic isolation of brain-derived EVs using TENPO.
A. The workflow of our machine learning-based TBI diagnostics that uses brain-derived EVs isolated using TENPO. B. EVs are immunomagnetically labeled using biotin anti-GluR2 antibody and anti-biotin magnetic nanoparticles (MNP). C. Finite element simulation of the magnetic field gradient generated by the TENPO’s permalloy-coated nanopores. The highest magnetic field gradient is at the edges of the pores where the labelled EVs are captured. D. The TENPO consists of millions of magnetic nanopores (600 nm diameter), which are incorporated into an acrylic reservoir. Fluid is pulled through the chip from the output using a syringe pump. The scale bar is 1 μm. E. Size distribution of EVs measured with DLS. Input is mixed cortical neuron/astrocyte cultured medium and isolate are GluR2+ EVs eluted from TENPO. The input has peaks at 8.7 nm, 78.8 nm, and 615 nm whereas the isolate has a major peak at 141.8 nm. F. Scanning electron microscopy (SEM) image of an EV captured using TENPO. The captured particle has a morphology consistent with EVs labeled with magnetic nanoparticles. G. Total EV miRNA yield (ng) comparison between centrifugation method (122.7 ng) and TENPO (216.2 ng). EVs are isolated from 20ml of mixed cortical neuron/astrocyte cultured medium.