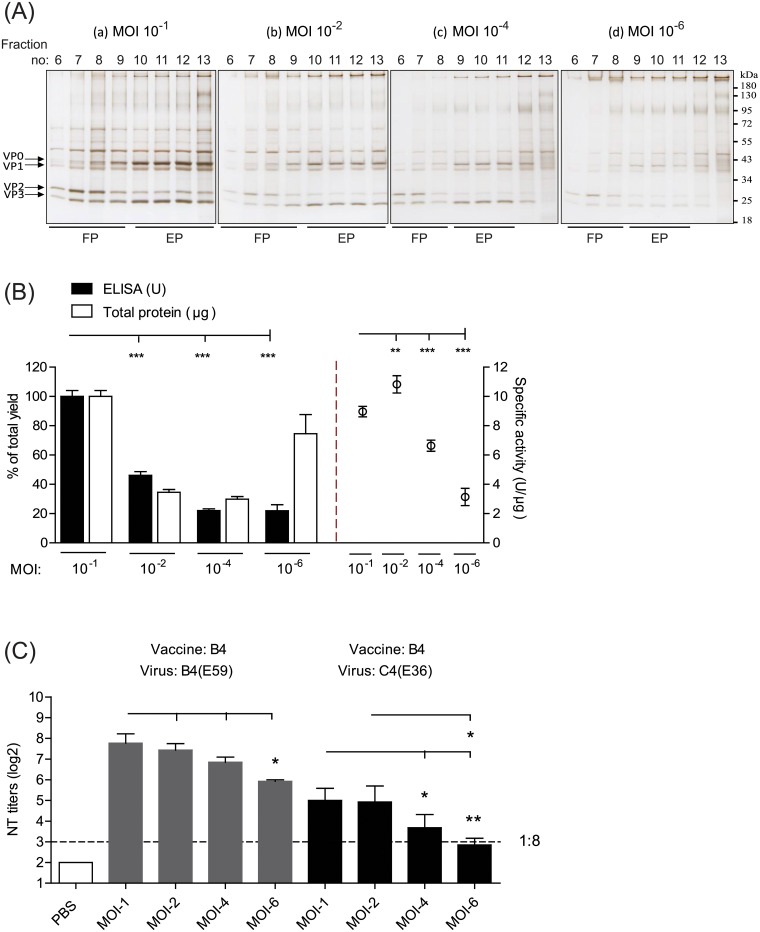
Fig 2. Productivity, specific activity, and immune potency of EV71 vaccines produced from different MOI cultures.
(A) Purification of EV71 antigens from different MOI cultures by sucrose density gradient ultracentrifugation (SDG) and identification of the viral particle-enriched fractions using SDS-PAGE analysis with silver staining. (a)-(d). EV71 cultures at different MOIs as indicated above. The lines labeled with FP and EP represent the distributed fractions of the infectious full particles and defective empty particles, respectively. The molecular weights of VP0, VP1, VP2, and VP3 are indicated. The molecular weight marker is shown on the right. (B) The ELISA yield, total protein, and specific activity of EV71 vaccines purified from different MOI cultures. After SDG purification, the EV71 particle-enriched fractions (fractions 6–13) were subsequently pooled and inactivated for productivity and quality evaluation. (C) Viral neutralization titers against the B4(E59) and C4(E36) subgenotypes elicited by EV71 vaccines from different MOI preparations. Significant differences between vaccine groups are indicated with the following symbols: *, p<0.05; **, p<0.01; and ***, p<0.001.