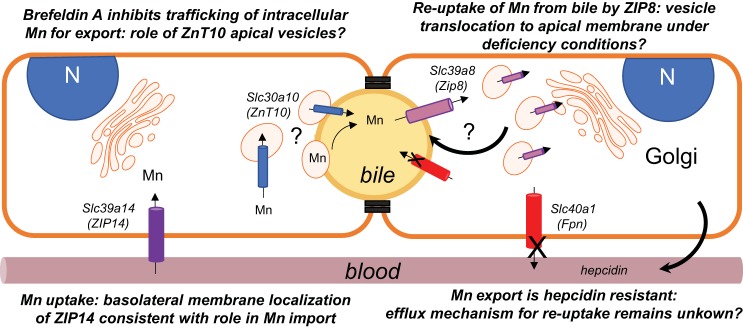
Fig. 7.
Summary model of polarized WIF-B hepatocyte manganese (Mn) transport and trafficking. Two adjacent WIF-B cells are depicted with polarity maintained by tight junctions to form basolateral (sinusoidal; orange) and apical (bile canalicular, yellow) membrane surfaces. Four transporters that are implicated in Mn transport were examined in this polarized hepatocyte model: ZIP14 (purple), ZnT10 (blue), ZIP8 (pink), and ferroportin (Fpn) (red). ZIP14 is observed to associate with the basolateral (sinusoidal) surface, consistent with its proposed role in Mn uptake. The exporter Fpn is also localized to the basolateral membrane, but Mn efflux from WIF-B cells was resistant to hepcidin despite downregulation of Fpn. This observation suggests other pathways may play a role in exporting the metal from hepatocytes. Such an Mn release pathway would be necessary for the proposed reabsorption of Mn by ZIP8 under deficiency conditions. Our study shows that ZIP8 is not localized to the apical membrane but instead localizes to vesicles distributed throughout the cytoplasm. These vesicles could place this transporter in the bile canalicular membrane upon trafficking induced by Mn deficiency. Like ZIP8, ZnT10 is also localized to intracellular vesicles, but these are directly adjacent to the bile canalicular (apical membrane); the transporter does not appear to be integrally associated with the bile canalicular surface. We observe that the secretory inhibitor brefeldin A reduces Mn export, suggesting that the ZnT10-positive apical vesicles may contain Mn and subsequently fuse with the bile canalicular membrane to empty the contents into bile. Alternatively, fusion of these vesicles with the bile canalicular membrane may place ZnT10 into the membrane, thereby accommodating Mn export by the exporter.