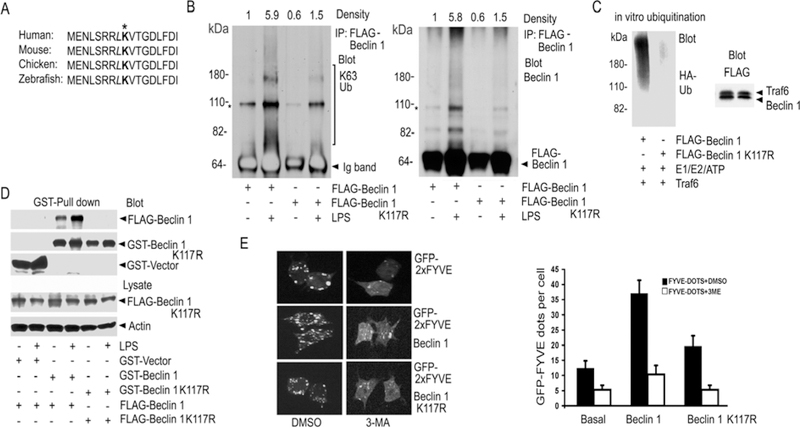
Fig. 5.
Mutation of Lysine 117 in Beclin 1 reduces Beclin 1 ubiquitination. (A) Amino acid alignment of a portion of the BH3 domains of different species. (B) Contrast between LPS-induced ubiquitination of Beclin 1 and its K117R mutant. FLAG immunoprecipitates of Raw 264.7 cells expressing FLAG-Beclin 1 or FLAG-K117R Beclin 1 were immunoblotted for K63 linked ubiquitin (K63 Ub), stripped, and re-blotted for Beclin 1. The major non-specific bands (*) are indicated. The heavy chain of the FLAG antibody was detected by the secondary antibody in the left panel and is labeled Ig band. The density of each lane over the indicated area is shown. (C) Beclin 1 K117R is poorly ubiquitinated in vitro. Purified FLAG-Beclin 1 or FLAG-Beclin 1 K117R was incubated with eluted FLAG-Traf6 along with reaction components. The reactions were immunoblotted for HA-Ub. Similar amounts of Traf6 and the FLAG-tagged proteins were in the reaction. (D) Beclin 1 ubiquitination enhances Beclin 1 oligomerization. GST-pull downs from Raw 264.7 cells expressing GST-Beclin 1 and FLAG-Beclin 1 or GST-Beclin 1 K117R and FLAG-Beclin 1 K117R stimulated with LPS, or not, were immunoblotted for FLAG. (E) Beclin 1, but not its K117R mutant, enhances the activity of PI3KC3 in Raw 264.7 cells. GFP-2xFYVE dots were imaged. Beclin 1 or Beclin 1 K117R was expressed in Raw 264.7 cells along with GFP-2xFYVE. The cells were imaged and then treated with 5mM 3-MA or DMSO for 2 h. The cells were then re-imaged. Representative cells are shown (top panel). The average number of dots in 100 cells from the indicated each sample was determined. Data displayed as mean +/− SD of the number of dots/cell (bottom panel). Similar results in 3 experiments performed.