Figure 2. The ability of BZA to disrupt Y537S and D538G ERα activity.
(A) Cell growth in MCF-7 cells with DOX-induced Y537S ERα expression. (B) Inhibition of ERα target genes in DOX-induced Y537S ERα expressed MCF-7 cells with increasing doses of BZA. (C) Representative immunoblot of HA-ERα WT, 537S, or D538G treated with E2, 4-OHT, BZA, RAL, or FULV for 24 hr. (D) Representative counts of HA-ERα from the immunoblot normalized to actin.
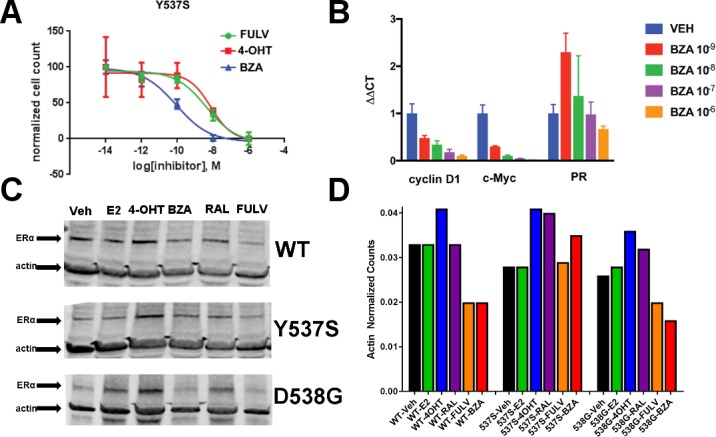
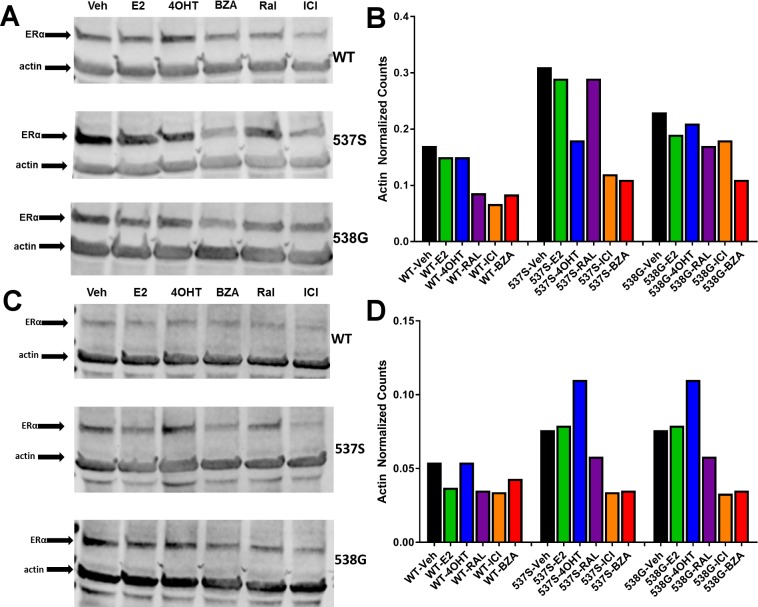
Figure 2—figure supplement 1. Replicate experiments of HA-ERα levels in MCF-7 cells upon treatment with E2, 4-OHT, RAL, fulvestrant (FULV), or BZA for 24 hr.
Figure 2—figure supplement 2. BZA-induced degradation of ERα in CAMA-1, MDA-MB-361, T47D, and ZR75-1 breast cancer cell lines.