Table 1. Competitive inhibitors of estrogen receptor alpha.
| Antiestrogen | Class | Approved clinical indications |
|---|---|---|
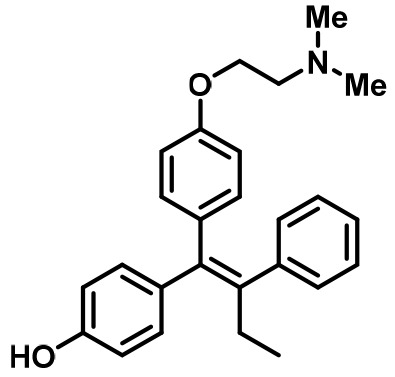 4-Hydroxytamoxifen (4-OHT) |
SERM | • Adjuvant treatment for ER + breast cancers (Early Breast Cancer Trialists' Collaborative Group., 1998). • Metastatic Breast Cancer (Lipton, 1982). • Ductal Carcinoma in Situ (Allred et al., 2012). • Reduction in Breast Cancer Incidence in High Risk Women (Visvanathan, 2009). |
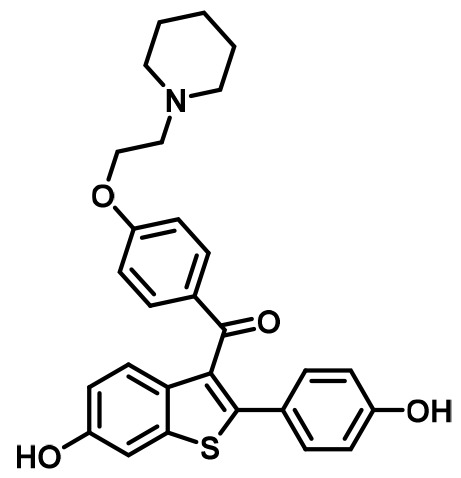 Raloxifene (RAL) |
SERM | • Osteoporosis in postmenopausal women (Messalli and Scaffa, 2010). • Reduction in Breast Cancer Incidence in High Risk Women(Cauley et al., 2001). |
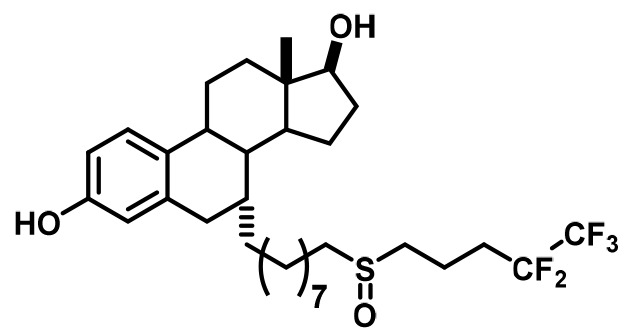 Fulvestrant (FULV) |
SERD | • First-line therapy for metastatic breast cancer (Howell et al., 2004). • Postmenopausal women with progressive breast cancer following other antiestrogen therapy (Osborne et al., 2002; Howell et al., 2002). |
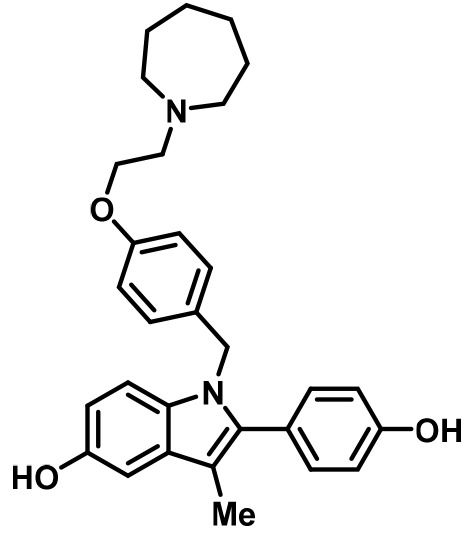 Bazedoxifene (BZA) |
SERM/SERD | • In combination with conjugated equine estrogens (DUAVEE) to prevent postmenopausal osteoporosis (Tikoo and Gupta, 2015). |
