FIGURE 9.
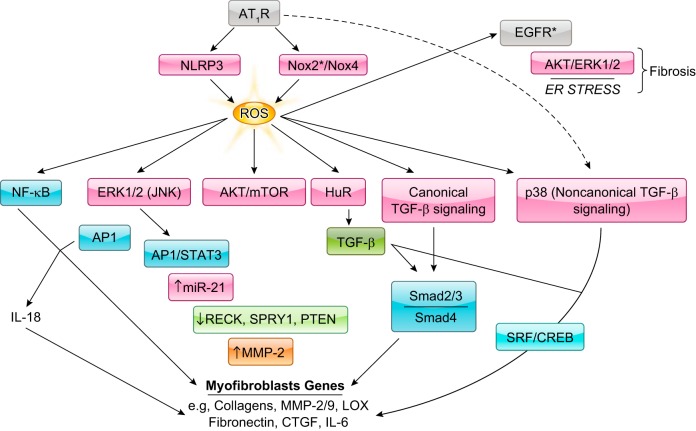
ROS has a critical role in ANG II-induced fibrosis of the heart. In the context of fibrosis, AT1R induces ROS formation primarily via engagement of Nox2 and Nox4, but also via mitochondria in a process involving the pattern recognition receptor NLRP3. ROS leads to the induction of various signaling pathways, including NF-κB, ERK1/2, and Akt/mTOR that are linked to myofibroblast target genes. NF-κB and AP1 are implicated in cardiac fibroblast proliferation, migration, and collagen production through the induction of IL-18. ERK1/2 activation is linked to both AP1 and STAT3 activation and the upregulation of miR-21, which in turn suppresses MMP regulator reversion-inducing cysteine-rich protein with Kazal motifs (RECK), Sprouty homologue 1 (SPRY1), and PTEN. Reduction of these proteins increases MMP-2 production or activity and encourages cardiac fibroblast migration. ANG II-induced ROS is implicated as well in both canonical and noncanonical TGF-β signaling. The former involves association of phosphorylated Smad2 and 3 with Smad4; the latter activation of SRF or CREB via p38MAPK signaling, which is engaged directly by an AT1R-linked cascade or indirectly through ROS formation. Increased TGF-β levels may contribute to ANG II-induced fibrosis because of the stabilization of TGF-β mRNA resulting from ROS-induced activation of RNA binding protein HuR. Lastly, EGFR transactivation may contribute to ANG II-induced fibrosis, although cardiac myocytes and not fibroblasts may be involved in this mechanism. *May involve additional cell types besides cardiac fibroblasts/myofibroblasts.