Abstract
Background
Esophageal adenocarcinoma (EA) and gastric cardia adenocarcinoma (GCA) are characterized by a strong male predominance. Concentrations of sex steroid hormones have been hypothesized to explain this sex disparity. However, no prospective population-based study has examined sex steroid hormones in relation to EA/GCA risk. Thus, we investigated whether prediagnostic circulating sex steroid hormone concentrations were associated with EA/GCA in a nested case–control study drawn from participants in three prospective cohort studies.
Methods
Using gas chromatography–mass spectrometry (GC-MS) and electrochemiluminescence immunoassay, we quantitated sex steroid hormones and sex hormone binding globulin, respectively, in serum from 259 EA/GCA male case participants and 259 matched male control participants from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, and Cancer Prevention Study II Nutrition Cohort. Multivariable conditional logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between circulating hormones and EA/GCA risk. All statistical tests were two-sided.
Results
Higher concentrations of dehydroepiandrosterone (DHEA) were associated with a 38% decreased risk of EA/GCA (OR per unit increase in log2 DHEA = 0.62, 95% CI = 0.47 to 0.82, Ptrend = .001). Higher estradiol concentrations were associated with a 34% reduced risk of EA/GCA (OR = 0.66, 95% CI = 0.45 to 0.98, Ptrend = .05), and the association with free estradiol was similar. No other associations between baseline hormone concentrations and future EA/GCA risk were observed.
Conclusions
This study provides the first evidence that higher concentrations of circulating DHEA, estradiol, and free estradiol may be associated with lower risks of EA/GCA in men.
Esophageal adenocarcinoma (EA) incidence has increased approximately 600% over the last 35 years in the United States, placing it among the most rapidly increasing cancer types (1,2). The incidence of anatomically linked gastric cardia adenocarcinoma (GCA) has also increased but less rapidly than EA (3). EA and GCA are often considered a singular clinical entity since they both occur at or near the gastroesophageal junction and have similar overall and stage-specific five-year survival rates (4).
Age-adjusted incidence of EA/GCA is four to eight times higher in men than women (5,6). These sex differences have been considered to be the result of established risk factors that differ in prevalence by sex, such as obesity, reflux, and smoking. However, analyses of these factors have explained very little of the male predominance of these tumors (7,8). Sex hormones may potentially account for this sex disparity (9,10). This hypothesis is supported by sex steroid hormone involvement in the inflammatory process (11–14); expression of estrogen receptors (ERs) in esophageal and gastric cancer tissue (9,15–20); lower rates of esophageal and gastric cancer among men with prostate cancer, who are likely to receive anti-androgen therapies (21–24); and in women, lower rates of esophageal and gastric cancer associated with reproductive factors and estrogen hormone replacement therapies (25–27). Additionally, a population-based case–control study found that androgen:estrogen balance was associated with increased odds of EA (44), and a small hospital-based study reported higher testosterone concentrations among EA case participants (n = 25) than control participants (n = 8) (28). One small prospective study reported lower concentrations of dehydroepiandrosterone (DHEA) and DHEA-sulfate among gastric cancer case participants (n = 13) than control participants (n = 52) (29), but the study did not report if any of the case participants were diagnosed with GCA.
To date, no study has been conducted of sex steroid hormones and EA/GCA risk with prediagnostically collected blood samples. Thus, we conducted a pooled, nested case–control study using prediagnostic serum samples from three large prospective cohort studies to evaluate associations between concentrations of circulating sex steroid hormones and future EA/GCA risks.
Methods
Study Population
The current study leveraged the resources from three prospective parent cohort studies: Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial; Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study; and Cancer Prevention Study II (CPS-II) Nutrition Cohort (30–32). These studies were combined in order to have a sufficient sample size to examine the rare outcome of EA/GCA. All study protocols were approved by the institutional review boards of the participating institutions, and written informed consent was obtained from participants.
The PLCO Trial was a randomized controlled trial conducted at 10 study sites to evaluate the effects of cancer screening on cancer-related mortality (30). Eligible participants were men and women, age 55–74 years, between 1993 and 2001. Exclusions included persons with prior prostate, lung, colon, rectum, or ovarian cancer or currently undergoing cancer treatment. Participants completed a self-administered baseline questionnaire, which included anthropometric measurements. The PLCO Trial included 154 901 participants (76 685 men, 78 216 women).
The ATBC Study was a randomized controlled trial among Finnish male smokers, designed to test the effects of α-tocopherol and β-carotene supplementation on cancer incidence (31). Eligible participants were men, age 50–69 years, who smoked at least five cigarettes per day at the time of study recruitment, between 1985 and 1988. Exclusions included persons with prior cancer or other conditions that would limit participation in the trial. Participants completed a self-administered baseline questionnaire, and anthropometric measurements were collected by trained nurses. The ATBC Study included 29 133 male participants.
The CPS-II Nutrition Cohort is a subset of participants in the baseline CPS-II cohort study, which was established in 1982 to evaluate associations between diet and other exposures in relation to the risks of cancer incidence and mortality (32). Eligible participants were men and women, age 50–74 years, residing in 21 states with population-based cancer registries, who completed a mailed self-administered questionnaire in 1992–1993, which included anthropometric measurements. Exclusions included persons with a prior self-reported cancer diagnosis. The CPS-II Nutrition Cohort included 162 408 participants (77 048 men, 85 360 women).
The current study was restricted to men because there were too few case participants among women to provide adequate statistical power. Eligible case participants with serum and a first primary invasive cancer diagnosis were classified, using ICD-O-3 (33) topography codes, as esophageal (C15.0-C15.9) and gastric cardia (C16.0). Only case participants with morphologies consistent with adenocarcinoma were included (8140–8575). Additionally, in the ATBC Study, adenocarcinomas have historically only been classified as esophageal if the tumor was 3 cm or more above the gastric junction and had no junctional involvement.
Control participants were matched one-to-one using incidence-density sampling based on study, age (±1 year), race/ethnicity, year of blood draw (±1 year), time of blood draw (am/pm), and number of freeze-thaw cycles for available serum samples. Thus, 202 participants are from the PLCO Trial, 248 from the ATBC Study, and 68 from the CPS-II Nutrition Cohort, providing a total of 259 EA/GCA case and 259 control participants.
Data and Sample Collection
The PLCO Trial collected blood samples from all participants in the screening intervention arm of the study during baseline visit; fasting status was not collected (34). The ATBC Study collected blood samples on all participants who had fasted for at least 12 hours at the time of their baseline clinic visit (31). The CPS-II Nutrition Cohort collected blood samples from approximately 39 000 CPS-II participants, who lived in urban and suburban areas, during a scheduled blood draw at a community hospital between 1998 and 2001; samples were generally nonfasting (32). Blood samples were frozen at –70°C in the PLCO Trial and ATBC Study (31,34), and at –130°C for the CPS-II Nutrition Cohort (32).
Laboratory Assays and Measurements
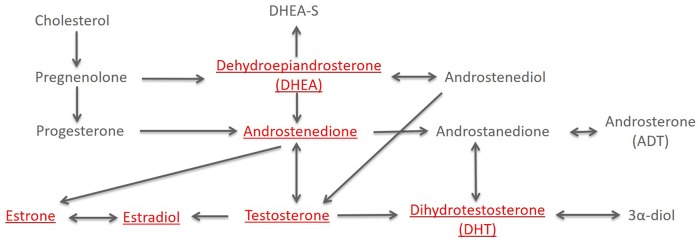
Sex steroid hormone assays were performed at the Pharmacogenomics Laboratory of Laval University (Quebec, Canada). Samples were quantitated for dehydroepiandrosterone (DHEA), androstenedione, testosterone, dihydrotestosterone (DHT), estrone, and estradiol using gas chromatography–mass spectrometry (GC-MS) (Figure 1) (35). In each of the 10 batches, three low– and three high–hormone concentration nonblinded quality control (QC) replicates were included. In these QCs, coefficients of variation (CVs) were less than 12% (range = 2.9%–11.9%). Additionally, approximately five blinded QCs were also included in each batch, and the CVs were less than 15% (range = 4.2%–14.7%). The exception to this was estrone (23.8%), but the CV was similar after batch 10 was excluded (14.1%). Excluding this batch from the analysis did not alter estimates of association (data not shown). Sample assays were excluded (ie, treated as missing values) if they were below the limit of quantification (eg, true low hormone concentration, poor chromatography or low internal standard; DHEA n = 3, androstenedione n = 2, testosterone n = 2, DHT n = 4, estrone n = 7, and estradiol n = 2).
Figure 1.
Schematic of sex steroid hormone metabolism. Quantitated sex steroid hormones are underlined. Sex hormone binding globulin is not shown, as it is not part of the sex steroid metabolism pathway.
Sex hormone binding globulin (SHBG) was quantitated at the Clinical and Epidemiologic Research Laboratory of Boston Children’s Hospital (Boston, MA) using a competitive electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics, Indianapolis, IN). In each of the 10 batches, approximately five blinded quality controls were included, and the CV was 4.2%.
In addition to individual hormones, we calculated parent estrogens (the sum of estrone and estradiol), testosterone:parent estrogens ratio, testosterone:estradiol ratio, androstenedione:estrone ratio, free estradiol (36), free testosterone (37), and free DHT (38). Hormone concentrations were categorized in quartiles, based on the distributions among the control participants. Tests of linear trend were performed based on the quartile-specific medians of the hormone concentrations. As continuous hormone values were right skewed, values were also log2 transformed, which corresponds to a doubling of circulating sex steroid hormone per one-unit increase.
Statistical Analysis
Differences in potential covariates between case and control participants were assessed using a Wilcoxon-Mann-Whitney test for continuous variables and chi-square/Fisher exact test for categorical variables. Mean hormone concentrations were adjusted for age and study, and the analysis of variance (ANOVA) test was used to compare case and control participants. Conditional logistic regression, based on the incidence-density matched case–control pairs, was used to calculate the odds ratios (ORs), as an estimate of relative risk, and corresponding 95% confidence intervals (CIs) for the associations between circulating hormones and EA/GCA risk. If covariates (39) were associated with 1) exposure in the general population (ie, control participants) and 2) outcome among the unexposed (ie, lowest quartile of sex steroid hormone concentration) (40), then the full model was evaluated both with and without the covariate of interest. Subsequently, if the log OR changed by 10% or more due to variable elimination, the variable was considered a confounder and retained (39). Final adjusted models included age (continuous), education (less than high school, high school/GED, some college/technical, college graduate), smoking status (never, current, former), number of cigarettes per day for current smokers (continuous), body mass index (BMI; 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), and diabetes (yes, no). Additionally, adjustment for BMI as a continuous measure instead of categorical had negligible effects (data not shown). We also examined alcohol consumption, marital status, aspirin use, and study-specific enrollment sites, but these did not meet the inclusion criteria. Effect measure modification of the relationships between log2 transformed hormone concentrations and EA/GCA by a priori selected variables of age, smoking, BMI, and diabetes was assessed using likelihood ratio tests (39).
We estimated Pearson pairwise correlations for each biomarker pair. This correlation matrix was then used to estimate the effective number of independent comparisons (41) and the corresponding adjusted statistical significance threshold for an alpha of .05 using matSpDlite (42,43). For the 14 main comparisons, the effective number of tests was estimated as 8.3, with a corresponding Sidak adjusted statistical significance threshold of .006. All tests were two-sided. Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC), and R Studio, version 1.0.153 (RStudio, Inc., Boston, MA).
Sensitivity Analysis
In addition to calculating quartiles based on the overall control participant distribution, we calculated study-specific quartiles. Then, tests of linear trend were performed based on the quartile-specific score of the hormone concentrations. To examine potential issues with reverse causation, we examined our results stratified by median follow-up time (ie, time between blood draw and cancer diagnosis), as individuals with undiagnosed underlying esophageal disease (eg, Barrett’s esophagus, low- or high-grade dysplasia) may have altered hormone concentrations. We also examined heterogeneity by site (ie, esophageal or gastric cardia) using unconditional logistic regression to calculate the ratio of the odds ratios (ROR). For individuals with a hormone assay that was below the lower limit of quantification, we conducted an analysis whereby we assigned half the lower limit of quantification for the hormone value. Finally, for outlier observations, defined as ±3 standard deviations on the log2 scale from mean hormone concentrations among control participants, we conducted an analysis excluding these observations (DHEA n = 3, androstenedione n = 2, testosterone n = 7, DHT n = 10, estrone n = 10, and estradiol n = 7).
Results
As shown in Table 1, case participants were more likely than control participants to have a BMI of 30 kg/m2 or higher (26.6% vs 14.3%, P < .001) and to be a former smoker at study baseline (35.1% vs 27.4%, P = .006). All individual hormones had lower mean concentrations in case participants than in control participants (eg, DHEA 5.67 nmol/L vs 6.90 nmol/L, P < .001).
Table 1.
Distributions of examined variables by case–control status
| Variable | Control participants (n = 259) | Case participants (n = 259) | P* |
|---|---|---|---|
| Mean age at baseline (SD), y | 61.0 (6.6) | 62.0 (6.6) | .98 |
| Caucasian race, No. (%) | 256 (98.8) | 256 (98.8) | 1.00 |
| Body mass index, No. (%) | |||
| 18.5–<25 kg/m2 | 95 (36.7) | 64 (24.7) | |
| 25–<30 kg/m2 | 123 (47.5) | 125 (48.3) | |
| ≥30 kg/m2 | 37 (14.3) | 69 (26.6) | |
| Missing | 4 (1.5) | 1 (0.4) | <.001 |
| Smoking status, No. (%) | |||
| Never | 54 (20.9) | 29 (11.2) | |
| Current | 134 (51.7) | 139 (53.7) | |
| Former | 71 (27.4) | 91 (35.1) | .006 |
| Cigarettes/d, No. (SD) | 20.0 (9.2) | 20.0 (9.5) | .61 |
| History of diabetes, No. (%) | 20 (7.7) | 22 (8.5) | .75 |
| Education, No. (%) | |||
| Less than high school | 45 (17.4) | 53 (20.5) | |
| High school or GED | 20 (7.7) | 25 (9.7) | |
| Some college/technical | 128 (49.4) | 134 (51.7) | |
| College graduate | 65 (25.1) | 47 (18.2) | |
| Missing | 1 (0.4) | 0 | .24 |
| Study, No. (%) | |||
| PLCO | 101 (39.0) | 101 (39.0) | |
| ATBC | 124 (47.9) | 124 (47.9) | |
| CPSII | 34 (13.1) | 34 (13.1) | 1.00 |
| Hormone concentrations, age- and study-adjusted mean (95% CI)† | |||
| DHEA, nmol/L | 6.90 (2.40 to 19.86) | 5.67 (1.97 to 16.29) | <.001 |
| Androstenedione, nmol/L | 3.38 (1.72 to 6.67) | 3.21 (1.63 to 6.33) | .002 |
| Testosterone, nmol/L | 16.37 (10.63 to 25.20) | 15.42 (10.02 to 23.75) | .16 |
| DHT, pmol/L | 1211.92 (725.09 to 2025.62) | 1132.80 (677.51 to 1894.02) | .17 |
| Estrone, pmol/L | 123.21 (89.02 to 170.54) | 121.03 (87.41 to 167.59) | .42 |
| Estradiol, pmol/L | 81.43 (68.76 to 96.44) | 76.53 (64.60 to 90.66) | .02 |
| SHBG, nmol/L | 55.70 (37.99 to 81.67) | 53.20 (36.28 to 78.02) | .22 |
| Parent estrogens, pmol/L | 207.69 (162.12 to 266.05) | 200.31 (156.31 to 256.70) | .15 |
| Testosterone: parent estrogens ratio | 80.52 (59.53 to 108.91) | 77.11 (57.01 to 104.29) | .40 |
| Androstenedione: estrone ratio | 27.61 (17.26 to 44.17) | 26.63 (16.66 to 42.58) | .29 |
| Testosterone: estradiol ratio | 204.12 (140.59 to 296.37) | 201.94 (139.09 to 293.19) | .91 |
| Free testosterone, nmol/L | 0.24 (0.18 to 0.31) | 0.23 (0.17 to 0.30) | .38 |
| Free DHT, pmol/L | 19.56 (13.85 to 27.60) | 19.06 (13.51 to 26.90) | .69 |
| Free estradiol, pmol/L | 1.78 (1.48 to 2.13) | 1.70 (1.42 to 2.03) | .09 |
Calculated using the Wilcoxon-Mann-Whitney test, chi-square/Fishers exact test, and analysis of variance test, as applicable.
All P values are from two-sided statistical tests. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CPSII = Cancer Prevention Study II Nutrition Cohort; DHEA = dehydroepiandrosterone; DHT = dihydrotestosterone; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; SHBG = sex hormone binding globulin.
Standardized to 61 years, mean age among control participants.
A doubling in the circulating concentration of DHEA was associated with a 38% reduced EA/GCA risk (OR per one-unit increase in log2 DHEA = 0.62, 95% CI = 0.47 to 0.82) (Table 2). Results were consistent when we examined quartiles of DHEA concentration (OR quartile 4 vs 1 = 0.28, 95% CI = 0.13 to 0.64, Ptrend = .001). Results for DHEA remained statistically significant after adjustment for multiple comparisons. A doubling in the circulating concentration of estradiol was associated with a 34% reduced EA/GCA risk (OR per one-unit increase in log2 estradiol = 0.66, 95% CI = 0.45 to 0.98). Results were consistent when we examined quartiles of estradiol concentration (OR quartile 4 vs 1 = 0.55, 95% CI = 0.31 to 0.99, Ptrend = .05). The association between free estradiol and EA/GCA was nearly identical to the estradiol-EA/GCA association. When we mutually adjusted for DHEA and estradiol, results were not notably altered (OR per one-unit increase in log2 DHEA = 0.64, 95% CI = 0.48 to 0.85, and OR per one-unit increase in log2 estradiol = 0.73, 95% CI = 0.49 to 1.11). No other associations were observed between hormones and EA/GCA risk. Additionally, no effect measure modification by age, smoking, BMI, or diabetes was observed (all P ≥ .05).
Table 2.
Adjusted* odds ratios and 95% confidence intervals for associations between circulating sex steroid hormone concentrations and esophageal and gastric cardia adenocarcinoma risk
| Hormone | Control participants, No. | Case participants, No. | OR (95% CI) |
|---|---|---|---|
| DHEA, nmol/L | |||
| <4.02 | 60 | 79 | Referent |
| 4.02 to <7.36 | 63 | 73 | 0.81 (0.46 to 1.45) |
| 7.36 to <12.26 | 64 | 58 | 0.5 (0.25 to 0.99) |
| ≥12.26 | 63 | 40 | 0.28 (0.13 to 0.64) |
| Ptrend† | .001 | ||
| Continuous (log2) | 250 | 250 | 0.62 (0.47 to 0.82) |
| Androstenedione, nmol/L | |||
| <2.40 | 62 | 73 | Referent |
| 2.40 to <3.32 | 60 | 42 | 0.56 (0.30 to 1.05) |
| 3.32 to <5.09 | 64 | 83 | 1.05 (0.55 to 2.01) |
| ≥5.09 | 65 | 53 | 0.71 (0.34 to 1.48) |
| Ptrend† | .93 | ||
| Continuous (log2) | 251 | 251 | 0.84 (0.57 to 1.22) |
| Testosterone, nmol/L | |||
| <12.65 | 62 | 78 | Referent |
| 12.65 to <16.70 | 62 | 58 | 0.77 (0.44 to 1.33) |
| 16.70 to <23.11 | 63 | 68 | 1.01 (0.58 to 1.76) |
| ≥23.11 | 64 | 47 | 0.64 (0.34 to 1.20) |
| Ptrend† | .47 | ||
| Continuous (log2) | 251 | 251 | 0.91 (0.71 to 1.16) |
| DHT, pmol/L | |||
| <923.19 | 62 | 78 | Referent |
| 923.19 to <1290.17 | 61 | 67 | 1.08 (0.63 to 1.85) |
| 1290.17 to <1743.03 | 63 | 46 | 0.63 (0.33 to 1.18) |
| ≥1743.03 | 63 | 58 | 0.86 (0.46 to 1.60) |
| Ptrend† | .67 | ||
| Continuous (log2) | 249 | 249 | 0.94 (0.71 to 1.24) |
| Estrone, pmol/L | |||
| <98.37 | 58 | 69 | Referent |
| 98.37 to <124.80 | 61 | 62 | 0.76 (0.42 to 1.36) |
| 124.80 to <158.98 | 65 | 52 | 0.58 (0.32 to 1.04) |
| ≥158.98 | 63 | 64 | 0.69 (0.39 to 1.24) |
| Ptrend† | .37 | ||
| Continuous (log2) | 247 | 247 | 0.82 (0.58 to 1.18) |
| Estradiol, pmol/L | |||
| <63.87 | 59 | 71 | Referent |
| 63.87 to <80.90 | 65 | 65 | 0.86 (0.49 to 1.52) |
| 80.90 to <103.15 | 63 | 68 | 0.89 (0.51 to 1.54) |
| ≥103.15 | 64 | 47 | 0.55 (0.31 to 0.99) |
| Ptrend† | .05 | ||
| Continuous (log2) | 251 | 251 | 0.66 (0.45 to 0.98) |
| SHBG, nmol/L | |||
| <40.93 | 62 | 68 | Referent |
| 40.93 to <57.92 | 65 | 76 | 0.98 (0.57 to 1.67) |
| 57.92 to <73.87 | 62 | 43 | 0.67 (0.35 to 1.26) |
| ≥73.87 | 64 | 66 | 1.05 (0.57 to 1.93) |
| Ptrend† | .94 | ||
| Continuous (log2) | 253 | 253 | 0.92 (0.66 to 1.28) |
| Parent estrogens, pmol/L | |||
| <168.83 | 57 | 69 | Referent |
| 168.83 to <208.17 | 65 | 70 | 0.78 (0.43 to 1.41) |
| 208.17 to <259.10 | 62 | 46 | 0.51 (0.28 to 0.91) |
| ≥259.10 | 63 | 62 | 0.66 (0.37 to 1.19) |
| Ptrend† | .11 | ||
| Continuous (log2) | 247 | 247 | 0.73 (0.49 to 1.09) |
| Testosterone: parent estrogens ratio | |||
| <58.40 | 64 | 58 | Referent |
| 58.40 to <85.33 | 60 | 84 | 1.82 (1.04 to 3.21) |
| 85.33 to <115.56 | 60 | 59 | 1.38 (0.78 to 2.45) |
| ≥115.56 | 61 | 44 | 1.03 (0.52 to 2.04) |
| Ptrend† | .94 | ||
| Continuous (log2) | 245 | 245 | 1 (0.77 to 1.31) |
| Androstenedione: estrone ratio | |||
| <20.62 | 60 | 64 | Referent |
| 20.62 to <27.32 | 63 | 55 | 0.89 (0.49 to 1.61) |
| 27.32 to <38.10 | 61 | 68 | 1.31 (0.69 to 2.49) |
| ≥38.10 | 62 | 59 | 1.19 (0.61 to 2.34) |
| Ptrend† | .54 | ||
| Continuous (log2) | 246 | 246 | 1.02 (0.73 to 1.43) |
| Testosterone: estradiol ratio | |||
| <153.02 | 65 | 63 | Referent |
| 153.02 to <204.60 | 61 | 62 | 1.09 (0.62 to 1.89) |
| 204.60 to <289.04 | 60 | 79 | 1.43 (0.81 to 2.52) |
| ≥289.04 | 63 | 45 | 0.89 (0.45 to 1.76) |
| Ptrend† | .63 | ||
| Continuous (log2) | 249 | 249 | 1.09 (0.79 to 1.51) |
| Free testosterone, nmol/L | |||
| <0.20 | 62 | 79 | Referent |
| 0.20 to <0.24 | 65 | 65 | 0.86 (0.50 to 1.46) |
| 0.24 to <0.30 | 60 | 57 | 0.83 (0.48 to 1.43) |
| ≥0.30 | 64 | 50 | 0.69 (0.38 to 1.25) |
| Ptrend† | .4 | ||
| Continuous (log2) | 251 | 251 | 0.91 (0.69 to 1.19) |
| Free DHT, pmol/L | |||
| <16.12 | 62 | 85 | Referent |
| 16.12 to <20.37 | 61 | 48 | 0.61 (0.34 to 1.07) |
| 20.37 to <25.53 | 63 | 49 | 0.61 (0.34 to 1.11) |
| ≥25.53 | 63 | 67 | 0.94 (0.53 to 1.63) |
| Ptrend† | .98 | ||
| Continuous (log2) | 249 | 249 | 1 (0.78 to 1.29) |
| Free estradiol, pmol/L | |||
| <1.43 | 61 | 65 | Referent |
| 1.43 to <1.74 | 63 | 71 | 0.99 (0.56 to 1.74) |
| 1.74 to <2.23 | 63 | 69 | 0.9 (0.51 to 1.57) |
| ≥2.23 | 64 | 46 | 0.56 (0.30 to 1.03) |
| Ptrend† | .04 | ||
| Continuous (log2) | 251 | 251 | 0.65 (0.43 to 0.99) |
Conditional logistic regression models were conditioned on matching factors (study, age, race/ethnicity, year of blood draw, and time of blood draw) and adjusted for age (continuous), education (less than high school, high school/GED, some college/technical, college graduate), smoking status (never, current, former), number of cigarettes per day (continuous), body mass index (18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), and diabetes (yes, no). CI = confidence interval; DHEA = dehydroepiandrosterone; DHT = dihydrotestosterone; OR = odds ratio; SHBG = sex hormone binding globulin.
Tests of linear trend were performed based on the quartile-specific medians of the hormone concentrations. All P values are from two-sided statistical tests.
Demographics and hormone concentrations by parent study are shown in Supplementary Table 1 (available online). As age, BMI, history of diabetes, and education level differed by parent study, we conducted a sensitivity analysis using study-specific quartiles of hormone concentrations. Results using these study-specific quartiles were similar to the main results (Supplementary Table 2, available online). For example, the highest vs lowest quartile of DHEA concentration was associated with a 62% reduced risk of EA/GCA (OR = 0.38, 95% CI = 0.21 to 0.70, Ptrend = .001), and estradiol was associated with a 38% reduced risk (OR = 0.62, 95% CI = 0.35 to 1.09, Ptrend = .05).
Adjustment for covariates had a minimal effect on the observed estimates, as evidenced in the minimally adjusted models (Supplementary Table 3, available online). When we stratified by time between blood draw and cancer diagnosis (< or ≥8.4 years), the results were similar (Supplementary Table 4, available online). We also examined the results by tumor site (ie, esophageal or gastric cardia) and found no notable heterogeneity, with the possible exceptions of DHT and free estradiol (Supplementary Table 5, available online). Finally, assigning half the lower limit of detection for missing hormone values or exclusion of outliers did not substantially alter the results (data not shown).
Discussion
Prediagnostic concentrations of circulating DHEA, estradiol, and free estradiol were associated with reduced risks (34%–38%) of EA/GCA in three large prospective cohort studies. No other associations were observed between hormones and EA/GCA risk.
This is the first study to examine the association between prediagnostic circulating sex steroid hormones and EA/GCA risk. While two previous studies have examined the association between circulating sex steroid hormones and EA risk, these studies collected blood samples at diagnosis (28,44). Thus, these studies are subject to potential reverse causation, whereby the tumor or other disease processes, such as cachexia, could affect concentrations of circulating sex steroid hormones. In the only prospective study of circulating sex steroid hormones and gastric cancer risk, the concentration of DHEA was lower among case compared with control participants (P = .09), but that study was small (13 case participants) and results for gastric cardia, specifically, were not reported (29). Additionally, two studies of the EA precursor metaplasia, Barrett’s esophagus, determined that higher concentrations of free androgens were associated with increased Barrett’s esophagus risk (45,46).
Concentrations of circulating sex steroid hormones observed in the current study were similar to previous reports for men (47–49). While circulating concentrations of DHEA-sulfate are known to be higher in men than in women (1280 ng/mL vs 610 ng/mL, age 55–60 years) (50–52), few studies have examined sex differences associated with circulating DHEA (52,53). Two studies have reported that concentrations of DHEA are higher in women than men (54,55), but one study reported similar concentrations of circulating DHEA by sex (56). Estradiol concentrations are known to be higher in women than in men until menopause, after which time estradiol concentrations are roughly equivalent (48).
Reasons for an inverse association between circulating DHEA concentration and EA/GCA risk are not completely understood. DHEA may enhance immune function (52) and has been inversely correlated with interleukin-6 (IL-6) (57), which can act as a pro-inflammatory cytokine. An in vitro model demonstrated that DHEA can inhibit IL-6 secretion from peripheral blood mononuclear cells (57). IL-6 has been implicated in EA development, with a recent study reporting that both mRNA and protein levels of IL-6 were overexpressed in gastro-esophageal cancer tissue specimens compared with normal epithelial tissue from control subjects (58). DHEA derivatives have been shown to exhibit inhibitory activity against gastric cancer cell lines (59). Additionally, a US Food and Drug Administration–approved chemotherapy agent for metastatic castration-resistant prostate cancer, abiraterone, functions as a selective inhibitor of CYP17 and suppresses serum DHEA concentrations by approximately 75% (60,61). Thus, DHEA could be reducing the EA/GCA risk through immunomodulation.
In men and women, ERs are expressed in esophageal and gastric tissue/cell lines (9,15–20). DHEA (62,63) and estradiol (64) are both full agonists of ER-β. Binding of this receptor by estradiol has been shown to inhibit cellular growth through induced apoptosis and cell cycle arrest (17)—a mechanism by which DHEA could also exert its independent effect, the greater impact of which may be attributable to its higher concentration and greater variability, relative to estradiol, in this male population. This mechanistic hypothesis is complicated by evidence that selective estrogen receptor modulators (ie, tamoxifen, raloxifene) (17,65) and antagonists (ie, MPP, PHTPP) (66) demonstrate similar antiproliferative effects. Estrone (67,68) and androstenedione (69) are less potent ER agonists, while testosterone (70) and DHT (71) exert action through the androgen receptor. These differential affinities for estrogen or androgen receptors may partially account for why we observed associations with estradiol and DHEA but not for other estrogens or androgens.
In older male populations, it has been shown that DHEA is inversely associated (72), and estradiol positively associated (73), with BMI. These prior studies align with our observations in the current study (DHEA-BMI ρ = −0.24, P = .01; estradiol-BMI ρ = 0.06, P = .53; free estradiol-BMI ρ = 0.20, P = .04), although these Spearman rank correlations evidence only weak relationships between these variables. These relationships are interesting but are unlikely to explain our results given that all analyses were adjusted for BMI, and there was no evidence of effect measure modification by BMI.
The strengths of this study include use of state-of-the-art quantitation of circulating sex steroid hormones and serum samples collected five to 12 years prior to incident diagnosis of cancer. Previous studies have utilized serum/plasma samples collected at the time of diagnosis (28,44), making it impossible to distinguish whether the observed hormone perturbations arose from the tumor or the disease process itself. Additionally, two of the previous studies that examined EA and gastric cancer utilized radioimmunoassay for hormone quantitation (28,29). While correlations between radioimmunoassay and mass spectrometry technologies are high, mass spectrometry is considered the gold standard for hormone quantification, as it has greater sensitivity and specificity (74).
Limitations of this study include the inability to assess circulating sex steroid hormones in women, lack of information on preexisting esophageal disease, and combining the outcomes of EA and GCA. Given that age-adjusted incidence of EA/GCA is four to eight times higher in men than women (5,6), pooled resources from the cohorts included in the current study, two of which recruited women, were still unable to provide a sufficient number of women participants for analysis. The three parent cohort studies included also provided no information on possible underlying esophageal diseases, which could affect the concentrations of circulating sex steroid hormones. Results between individuals diagnosed early in study follow-up (<8.4 years) and late in follow-up (≥8.4 years) were similar, suggesting that preexisting esophageal conditions did not substantially impact our findings.
In our main analysis, we combined the tumor sites of EA and GCA. However, there is some controversy as to whether they should be considered a combined outcome (75), and sex disparities do differ by site (5,6,31). However, we did stratify the results by tumor site, and the results did not indicate notable heterogeneity. Tumors that involve the distal esophagus and gastric cardia are difficult to classify, due to their close anatomic proximity (76). Thus, results stratified by site should be interpreted with caution, as there was heterogeneity among the three studies with regard to how tumors were classified as EA or GCA and there was no additional histopathologic review to attempt to further classify tumor site (77). Furthermore, there was heterogeneity by study in the concentrations of circulating sex steroid hormones. Therefore, we examined study-specific quartiles of circulating sex steroid hormones; these study-specific analyses were similar to the overall pooled results.
In summary, we report that DHEA, estradiol, and free estradiol are inversely associated with EA/GCA risk. While we were unable to assess the association between circulating sex steroid hormones and EA/GCA risk in women, the associations between DHEA and estradiol may partially explain the sex disparities seen in rates of EA and GCA. Before definitive conclusions can be made, these findings need to be replicated in a study population that includes women. However, due to the rarity of EA/GCA in women, this will require multi-institutional collaborations.
Funding
Supported by the National Institutes of Health Intramural Research Program, National Cancer Institute.
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (JLP, PLH, RTF, RMP, SMD, CCA, PRT, SJW, DA, NDF, MBC); Pharmacogenomics Laboratory, Centre Hospitalier de l’Université Laval de Québec (CHU de Québec) Research Center and Faculty of Pharmacy, Laval University, Québec, Canada (PC, CG); Epidemiology Research Program, American Cancer Society, Atlanta, GA (SMG, PTC); Clinical and Epidemiologic Research Laboratory, Department of Laboratory Medicine, Boston Children’s Hospital, Boston, MA (GB).
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to report.
Supplementary Material
References
- 1. Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;622:118–128. [DOI] [PubMed] [Google Scholar]
- 2. Pohl H, Welch HG.. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;972:142–146. [DOI] [PubMed] [Google Scholar]
- 3. Devesa SS, Blot WJ, Fraumeni JF Jr.. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;8310:2049–2053. [PubMed] [Google Scholar]
- 4. Wijnhoven BP, Siersema PD, Hop WC, et al. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;864:529–535. [DOI] [PubMed] [Google Scholar]
- 5. Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;6412:1881–1888. [DOI] [PubMed] [Google Scholar]
- 6. Xie SH, Lagergren J.. A global assessment of the male predominance in esophageal adenocarcinoma. Oncotarget. 2016;725:38876–38883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freedman ND, Derakhshan MH, Abnet CC, et al. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer. 2010;4613:2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutegard M, Nordenstedt H, Lu Y, et al. Sex-specific exposure prevalence of established risk factors for oesophageal adenocarcinoma. Br J Cancer. 2010;1035:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang H, Sukocheva OA, Hussey DJ, et al. Estrogen, male dominance and esophageal adenocarcinoma: Is there a link? World J Gastroenterol. 2012;185:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandanos E, Lagergren J.. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;4416:2397–2403. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt M, Naumann H, Weidler C, et al. Inflammation and sex hormone metabolism. Ann N Y Acad Sci. 2006;1069:236–246. [DOI] [PubMed] [Google Scholar]
- 12. Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):116–119. [PubMed] [Google Scholar]
- 13. Liao CH, Li HY, Yu HJ, et al. Low serum sex hormone-binding globulin: Marker of inflammation? Clin Chim Acta. 2012;413(7–8):803–807. [DOI] [PubMed] [Google Scholar]
- 14. Kupelian V, Chiu GR, Araujo AB, et al. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf). 2010;724:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rashid F, Khan RN, Iftikhar SY.. Probing the link between oestrogen receptors and oesophageal cancer. World J Surg Oncol. 2010;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuyama S, Ohkura Y, Eguchi H, et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;1286:319–324. [DOI] [PubMed] [Google Scholar]
- 17. Sukocheva OA, Wee C, Ansar A, et al. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Dis Esophagus. 2013;266:628–635. [DOI] [PubMed] [Google Scholar]
- 18. Liu L, Chirala M, Younes M.. Expression of estrogen receptor-beta isoforms in Barrett's metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004;24(5A):2919–2924. [PubMed] [Google Scholar]
- 19. Akgun H, Lechago J, Younes M.. Estrogen receptor-beta is expressed in Barrett's metaplasia and associated adenocarcinoma of the esophagus. Anticancer Res. 2002;223:1459–1461. [PubMed] [Google Scholar]
- 20. Kalayarasan R, Ananthakrishnan N, Kate V, et al. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;214:298–303. [DOI] [PubMed] [Google Scholar]
- 21. Cooper SC, Croft S, Day R, et al. Patients with prostate cancer are less likely to develop oesophageal adenocarcinoma: Could androgens have a role in the aetiology of oesophageal adenocarcinoma? Cancer Causes Control. 2009;208:1363–1368. [DOI] [PubMed] [Google Scholar]
- 22. Cooper SC, Trudgill NJ.. Subjects with prostate cancer are less likely to develop esophageal cancer: Analysis of SEER 9 registries database. Cancer Causes Control. 2012;236:819–825. [DOI] [PubMed] [Google Scholar]
- 23. McMaster ML, Feuer EJ, Tucker MA.. New malignancies following cancer of the male genital tract In: Curtis RE, Freedman DM, Ron E, et al. , eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. NIH Publ. No. 05-5302. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 24. Davis EJ, Beebe-Dimmer JL, Yee CL, et al. Risk of second primary tumors in men diagnosed with prostate cancer: A population-based cohort study. Cancer. 2014;12017:2735–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cronin-Fenton DP, Murray LJ, Whiteman DC, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: A pooled analysis. Eur J Cancer. 2010;4611:2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green J, Czanner G, Reeves G, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. 2012;13010:2387–2396. [DOI] [PubMed] [Google Scholar]
- 27. Lagergren K, Lagergren J, Brusselaers N.. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: A systematic review and meta-analysis. Int J Cancer. 2014;1359:2183–2190. [DOI] [PubMed] [Google Scholar]
- 28. Awan AK, Iftikhar SY, Morris TM, et al. Androgen receptors may act in a paracrine manner to regulate oesophageal adenocarcinoma growth. Eur J Surg Oncol. 2007;335:561–568. [DOI] [PubMed] [Google Scholar]
- 29. Gordon GB, Helzlsouer KJ, Alberg AJ, et al. Serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate and the risk of developing gastric cancer. Cancer Epidemiol Biomarkers Prev. 1993;21:33–35. [PubMed] [Google Scholar]
- 30. Kramer BS, Gohagan J, Prorok PC, et al. A National Cancer Institute sponsored screening trial for prostatic, lung, colorectal, and ovarian cancers. Cancer. 1993;71(2 Suppl):589–593. [DOI] [PubMed] [Google Scholar]
- 31. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;41:1–10. [DOI] [PubMed] [Google Scholar]
- 32. Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: Rationale, study design, and baseline characteristics. Cancer. 2002;949:2490–24501. [DOI] [PubMed] [Google Scholar]
- 32. Fritz AG. International Classification of Diseases for Oncology. 3rd ed, first revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 34. Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):349S–355S. [DOI] [PubMed] [Google Scholar]
- 35. Caron P, Turcotte V, Guillemette C.. A chromatography/tandem mass spectrometry method for the simultaneous profiling of ten endogenous steroids, including progesterone, adrenal precursors, androgens and estrogens, using low serum volume. Steroids. 2015;104:16–24. [DOI] [PubMed] [Google Scholar]
- 36. Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;166:801–810. [DOI] [PubMed] [Google Scholar]
- 37. Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;8410:3666–3672. [DOI] [PubMed] [Google Scholar]
- 38. Starka L, Pospisilova H, Hill M.. Free testosterone and free dihydrotestosterone throughout the life span of men. J Steroid Biochem Mol Biol. 2009;116(1–2):118–120. [DOI] [PubMed] [Google Scholar]
- 39. Rothman KJ, Greenland S, Lash TL.. Modern Epidemiology. 3rd ed Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 40. Dales LG, Ury HK.. An improper use of statistical significance testing in studying covariables. Int J Epidemiol. 1978;74:373–375. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Ji L.. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 2005;953:221–227. [DOI] [PubMed] [Google Scholar]
- 42. Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;744:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nyholt DR. Matrix Spectral Decomposition (matSpDlite). https://neurogenetics.qimrberghofer.edu.au/matSpDlite/. Accessed February 21, 2018.
- 44. Petrick JL, Falk RT, Hyland PL, et al. Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study. PLoS One. 2018;131:e0190325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cook MB, Wood SN, Cash BD, et al. Association between circulating levels of sex steroid hormones and Barrett's esophagus in men: A case-control analysis. Clin Gastroenterol Hepatol. 2015;134:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cook MB, Wood SN, Hyland PL, et al. Sex steroid hormones in relation to Barrett’s esophagus: An analysis of the FINBAR Study. Andrology. 2017;52:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gates MA, Mekary RA, Chiu GR, et al. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;986:2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yen SSC, Jaffe RB, Barbieri RL.. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 4th ed Philadelphia: Saunders; 1999. [Google Scholar]
- 49. Trabert B, Graubard BI, Nyante SJ, et al. Relationship of sex steroid hormones with body size and with body composition measured by dual-energy X-ray absorptiometry in US men. Cancer Causes Control. 2012;2312:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;593:551–555. [DOI] [PubMed] [Google Scholar]
- 51. Watson RR, Huls A, Araghinikuam M, et al. Dehydroepiandrosterone and diseases of aging. Drugs Aging. 1996;94:274–291. [DOI] [PubMed] [Google Scholar]
- 52. Kroboth PD, Salek FS, Pittenger AL, et al. DHEA and DHEA-S: A review. J Clin Pharmacol. 1999;394:327–348. [DOI] [PubMed] [Google Scholar]
- 53. Rehman KS, Carr BR.. Sex differences in adrenal androgens. Semin Reprod Med. 2004;224:349–360. [DOI] [PubMed] [Google Scholar]
- 54. Sulcova J, Hill M, Hampl R, et al. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol. 1997;1541:57–62. [DOI] [PubMed] [Google Scholar]
- 55. Carlstrom K, Brody S, Lunell NO, et al. Dehydroepiandrosterone sulphate and dehydroepiandrosterone in serum: Differences related to age and sex. Maturitas. 1988;104:297–306. [DOI] [PubMed] [Google Scholar]
- 56. Labrie F, Belanger A, Cusan L, et al. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;828:2396–2402. [DOI] [PubMed] [Google Scholar]
- 57. Straub RH, Konecna L, Hrach S, et al. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: Possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab. 1998;836:2012–2017. [DOI] [PubMed] [Google Scholar]
- 58. Deans DA, Wigmore SJ, Gilmour H, et al. Elevated tumour interleukin-1beta is associated with systemic inflammation: A marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006;9511:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ke S, Wei Y, Shi L, et al. Synthesis of novel steroid derivatives derived from dehydroepiandrosterone as potential anticancer agents. Anticancer Agents Med Chem. 2013;138:1291–1298. [DOI] [PubMed] [Google Scholar]
- 60. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;36421:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;2628:4563–4571. [DOI] [PubMed] [Google Scholar]
- 62. Webb SJ, Geoghegan TE, Prough RA, et al. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38(1–2):89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen F, Knecht K, Birzin E, et al. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;14611:4568–4576. [DOI] [PubMed] [Google Scholar]
- 64. Dahlman-Wright K, Cavailles V, Fuqua SA, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;584:773–81. [DOI] [PubMed] [Google Scholar]
- 65. Due SL, Watson DI, Bastian I, et al. Tamoxifen enhances the cytotoxicity of conventional chemotherapy in esophageal adenocarcinoma cells. Surg Oncol. 2016;253:269–277. [DOI] [PubMed] [Google Scholar]
- 66. Al-Khyatt W, Tufarelli C, Khan R, et al. Selective oestrogen receptor antagonists inhibit oesophageal cancer cell proliferation in vitro. BMC Cancer. 2018;181:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuhl H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. [DOI] [PubMed] [Google Scholar]
- 68. Escande A, Pillon A, Servant N, et al. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem Pharmacol. 2006;7110:1459–1469. [DOI] [PubMed] [Google Scholar]
- 69. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;1383:863–870. [DOI] [PubMed] [Google Scholar]
- 70. Nieschlag E, Behre HM, Nieschlag S.. Testosterone: Action, Deficiency, Substitution. 4th ed Cambridge: Cambridge University Press; 2012. [Google Scholar]
- 71. Mozayani A,, Raymon LP.. Handbook of Drug Interactions: A Clinical and Forensic Guide. New York: Humana Press; 2012. [Google Scholar]
- 72. Tchernof A, Labrie F.. Dehydroepiandrosterone, obesity and cardiovascular disease risk: A review of human studies. Eur J Endocrinol. 2004;1511:1–14. [DOI] [PubMed] [Google Scholar]
- 73. Muller M, den Tonkelaar I, Thijssen JH, et al. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003;1496:583–589. [DOI] [PubMed] [Google Scholar]
- 74. Hsing AW, Stanczyk FZ, Belanger A, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;165:1004–1008. [DOI] [PubMed] [Google Scholar]
- 75. Hayakawa Y, Sethi N, Sepulveda AR, et al. Oesophageal adenocarcinoma and gastric cancer: Should we mind the gap? Nat Rev Cancer. 2016;165:305–318. [DOI] [PubMed] [Google Scholar]
- 76. Misumi A, Murakami A, Harada K, et al. Definition of carcinoma of the gastric cardia. Langenbecks Arch Chir. 1989;3744:221–226. [DOI] [PubMed] [Google Scholar]
- 77. Ichihara S, Uedo N, Gotoda T.. Considering the esophagogastric junction as a ‘zone.’ Dig Endosc. 2017;29(Suppl 2):3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.