Scheme 1.
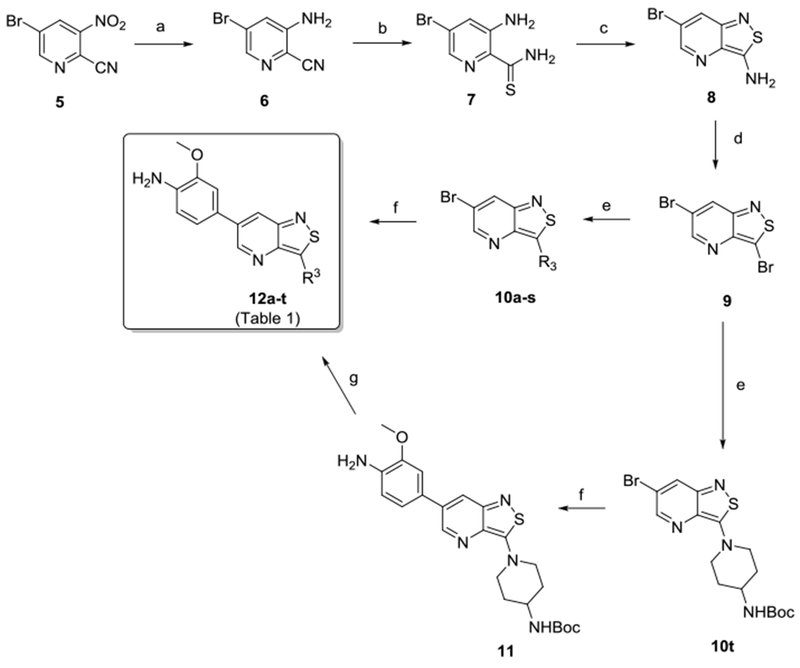
Reagents and conditions: a) Fe, CH3COOH, rt; b) Lawesson’s reagent, EtOH, reflux; c) 30% aq. H2O2, MeOH, 0 °C; d) NaNO2, HBr, CuBr, H2O, 0 °C to rt; e) R3H, EtOH or n-BuOH, reflux; f) 4-amino-3-methoxyphenylboronic acid pinacol ester, K2CO3, Pd(PPh3)4, dioxane/water, 100 °C; g) 37% HCl, dioxane, rt.
