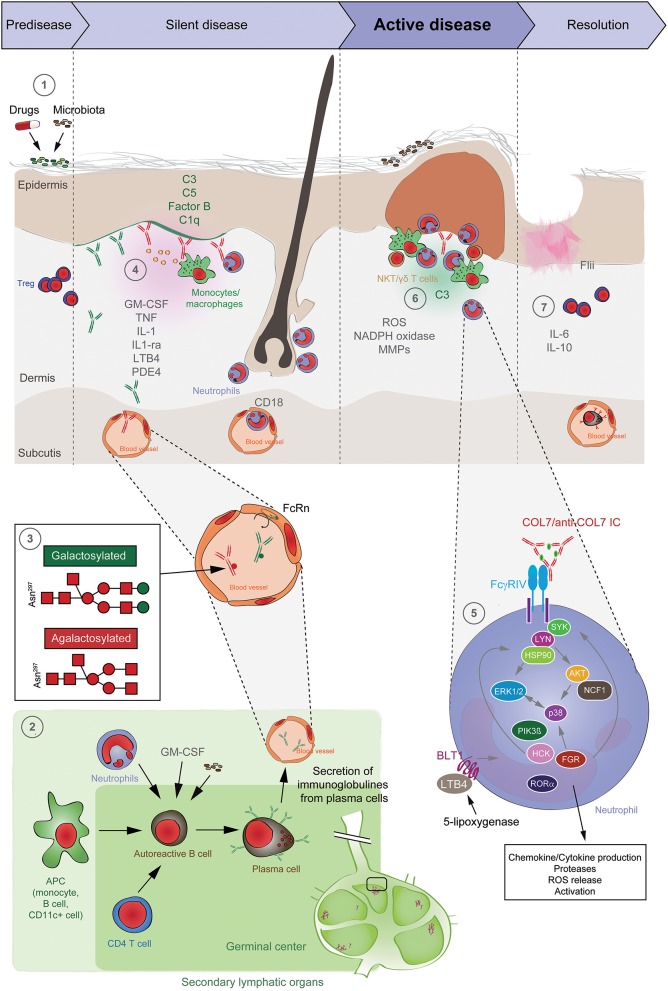
Figure 5.
Pathogenesis of EBA. (1) Genetic factors and the skin microbiome promote a tolerance loss. (2) This phenomenon is mediated by the interaction of APCs with autoreactive B and T cells, leading to clonal expansion and differentiation into plasma cells. Autoantibodies against COL7 are released into the blood circulation and effector organs. (3) During inflammation, galactosylation of antibodies may differ. High galactosylation of IgG is crucial for these anti-inflammatory properties, whereas low galactosylation is pro-inflammatory. (4) Binding of autoantibodies to DEJ in the skin induces complement deposition, pro-inflammatory cytokine and mediator release and subsequently leukocyte extravasation. (5) Immune complexes bind in a Fc-dependent manner to neutrophils and induce a signaling cascade leading to activation, including the (6) release of ROS and matrix metalloproteases. In addition to neutrophils, other cell types are involved in split formation, as shown for monocytes/macrophages, NKT and γδ T cells. By contrast, Treg cells have an inhibitory effect on EBA progression. (7) Resolution of autoantibody-induced tissue injury. Treg, regulatory T cell; NKT, natural killer cell; C, complement; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; LTB4, leukotriene B4; PDE4, phosphodiesterase 4; ROS, reactive oxygen species; NADPH, nicotinamide adenine dinucleotide phosphate; MMPs, matrix metalloproteinases; APC, antigen-presenting cell; CD, cluster of differentiation; SYK, spleen tyrosine kinase; Lyn, tyrosine-Protein Kinase Lyn; HSP, heat shock protein; AKT, protein kinase B; NCF1, neutrophil cytosolic factor 1; ERK, extracellular signal-regulated kinase; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; HCK, tyrosine-protein kinase HCK; FGR, tyrosine-protein kinase FGR; RORα, retinoid-related orphan receptor-alpha; BLT1, leukotriene B4 receptor 1; LTB4, leukotriene B4.