Fig. 2.
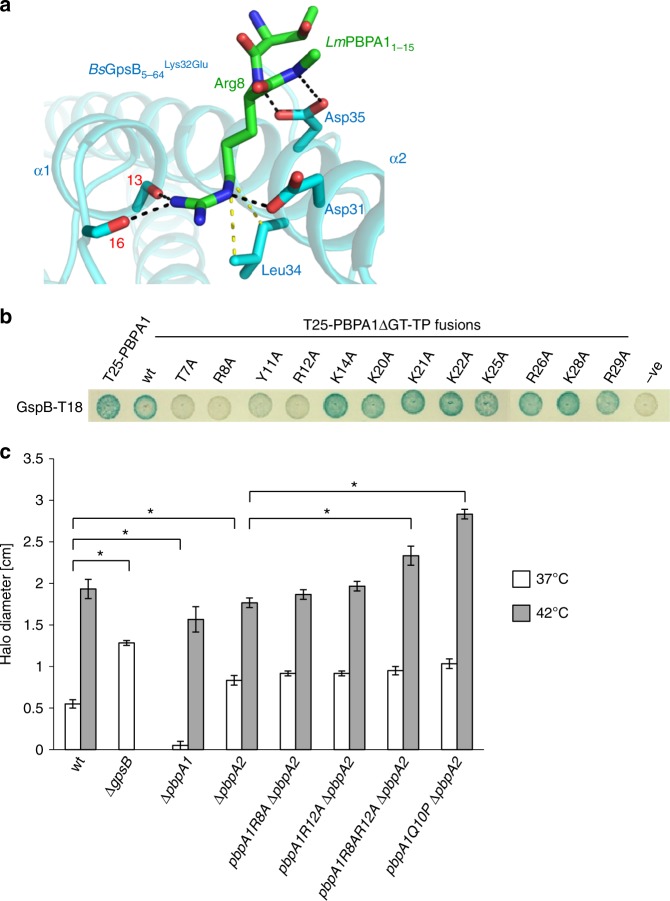
The LmGpsB:LmPBPA1 interactions are also governed by a conserved arginine. a The structure of the BsGpsB5-64Lys32Glu:LmPBPA11-15 complex reveals that only Arg8 and adjacent backbone atoms of the LmPBPA11-15 peptide are ordered. In this cartoon, BsGpsB5-64Lys32Glu is coloured cyan and selected sidechains are drawn as stick with cyan carbons, whereas the LmPBPA11-15 peptide is represented in stick form, with green carbons. The carbonyl oxygens of BsGpsBIle13 and BsGpsBLys16 are denoted by respective red numerals. Hydrogen bonds are shown as black dashed lines and the van der Waals’ interactions between BsGpsBLeu34 and LmPBPA1Arg8 are in yellow. Only one PBP-binding site is occupied by peptide in these crystals because the second site is blocked by crystal contacts. b Mutation of conserved LmPBPA1 residues results in a loss of interaction by BACTH. The removal of residues 92–827, correlating to the glycosyltransferase and transpeptidase domains of LmPBPA1, results in the PBPA1ΔGT-TP peptide. Empty pKT25 (−) was used as a negative control. Agar plates were photographed after 24 h at 30 °C. c Effect of N-terminal pbpA1 mutations on fosfomycin sensitivity of a ΔpbpA2 mutant. Wild-type and mutant L. monocytogenes EGD-e strains were grown as confluent layers on BHI agar plates at 37 °C and 42 °C and halo diameters around fosfomycin-containing filter discs were measured and corrected for the disc diameter. The experiment was performed in triplicate, and average values and standard deviations are shown. Asterisks indicate statistically significant differences (P < 0.01, t-test)