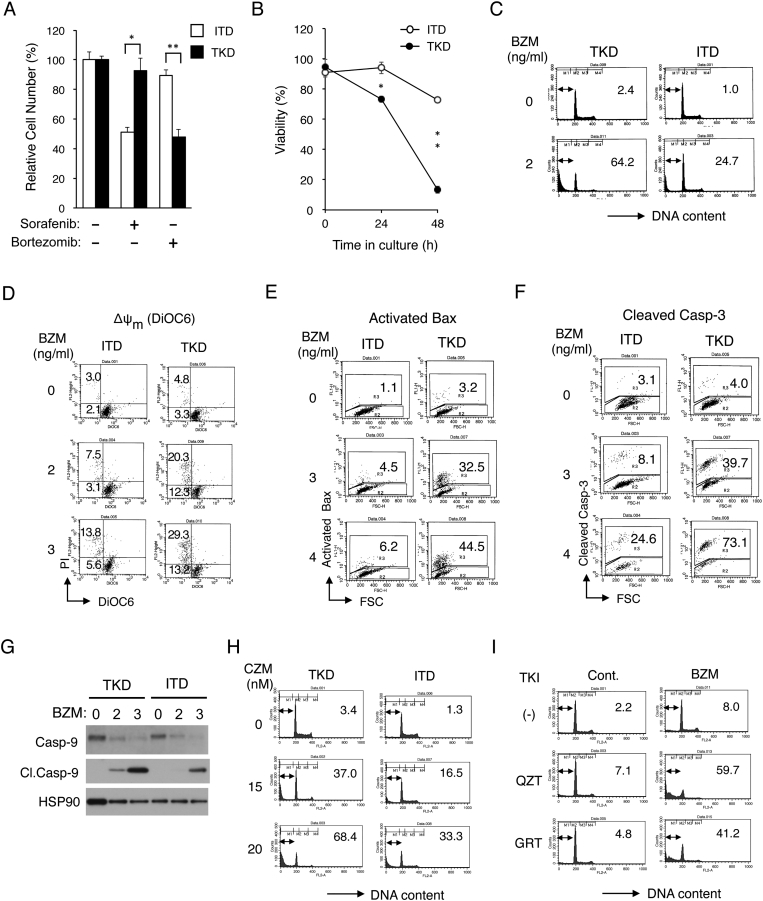
Figure 1.
FLT3-ITD confers resistance to bortezomib by inhibiting activation of the mitochondria-mediated intrinsic apoptotic pathway involving activation of Bax and caspases. (A) 32D/ITD (ITD) or 32D/TKD (TKD) cells were cultured for 48 hours with or without 50 nM sorafenib or 3 ng/ml bortezomib, as indicated. The means of relative viable cell numbers from triplicate measurements are plotted as percentages of control cells without inhibitors, with error bars indicating standard errors. The asterisks indicate a statistically significant difference determined by Student's t test (*P < .05, *P < .005). (B) Cells indicated were cultured with 3 ng/ml bortezomib for indicated times, and viable cell numbers were counted after trypan blue staining. The means of percentages of viable cells from triplicate measurements are shown, with error bars indicating standard errors (*P < .001, *P < .0001). (C) Cells indicated were treated for 48 hours with or without 2 ng/ml bortezomib (BZM), as indicated, and analyzed for the cellular DNA content by flow cytometry. Percentages of apoptotic cells with sub-G1 DNA content are indicated. (D) Cells indicated were cultured for 24 hours with BZM, as indicated, and analyzed for the mitochondrial membrane potential (∆ψm) using DiOC6 by flow cytometry. Percentages of cells with reduced ∆ψm stained with or without PI are indicated. (E, F) Cells indicated were cultured for 24 hours with BZM, as indicated, and analyzed for activated Bax (E) and cleaved Caspase-3 (F) by flow cytometry. Percentages of cells with activated Bax or cleaved Caspase-3 are indicated. FSC: forward scatter. (G) Cells indicated were treated with indicated concentrations (ng/ml) of BZM for 42 hours and subjected to Western blot analysis with antibodies against indicated proteins. Abbreviations: Casp-9, Caspase-9; Cl.Casp-9, cleaved Caspase-9. HSP90 was used for the loading control. (H) Cells indicated were treated for 48 hours with indicated concentrations of carfilzomib (CZM) and analyzed. (I) 32D/ITD cells were treated for 48 hours with or without 1 ng/ml BZM in the presence or absence of 0.5 nM quizartinib (QZT) or 5 nM gilteritinib (GRT), as indicated, and analyzed.