Abstract
Background
Echocardiography and 24‐hour ECG are the gold standard tests to diagnose dilated cardiomyopathy (DCM) in Doberman Pinschers (DP), but myocardial damage might be detected earlier using a high‐sensitivity cardiac troponin I (hs‐cTnI) assay.
Objective
To evaluate and compare an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay) with a conventional cTnI assay in DP with different stages of DCM and in healthy DP.
Animals
Three hundred forty‐five examinations from 162 DP with and 179 DP without DCM.
Methods
Prospective longitudinal study. Dogs were allocated into 6 groups based on echocardiographic and 24‐hour ECG criteria: (1) healthy group (179 dogs), (2) last‐normal group (29 dogs), which included dogs that were considered to be healthy at the time of their examination but were assigned to the last‐normal group retrospectively when DCM was diagnosed at their next examination within 1.5 years, (3) only arrhythmias (45 dogs, 119 examinations), (4) only echocardiographic changes (24 dogs, 61 examinations), (5) echocardiographic changes with ventricular premature complexes (41 dogs, 100 examinations), and (6) decompensated (23 dogs, 36 examinations). Hs‐cTnI and conventional cTnI concentration measurements were performed and compared.
Results
A cutoff value of hs‐cTnI concentration >0.113 ng/mL had a sensitivity of 81.2% and a specificity of 73.2% to identify the presence of DCM. The conventional cTnI assay showed a similar test performance, but the hs‐cTnI assay identified more dogs (21/29 dogs, 72%) in the last‐normal group compared to the conventional cTnI test (18/29 dogs, 62%).
Conclusions and Clinical Importance
The hs‐cTnI is an additional test with good potential to identify early DCM.
Keywords: Advia Centaur TnI‐Ultra assay, biomarker, cTnI, DCM, Doberman cardiomyopathy, high‐sensitivity cTnI assay
Abbreviations
- AUC
area under the curve
- BSA
body surface area
- CHF
congestive heart failure
- CI0.95
95% confidence interval
- CV
coefficient of variation
- DCM
dilated cardiomyopathy
- DP
Doberman Pinschers
- EDTA
ethylendiaminetetraacetic acid
- hs‐cTnI
high‐sensitivity cTnI
- LVEDV
left ventricular end‐diastolic volume
- LVESV
left ventricular end‐systolic volume
- ROC
receiver operator curves
- SMOD
Simpson's method of disc
- VPC
ventricular premature complexes
1. INTRODUCTION
Dilated cardiomyopathy (DCM) is an inherited, common cardiac disease of Doberman Pinschers (DP).1, 2, 3 The slowly progressing disease is characterized by 3 stages. Doberman Pinschers in stage 1 are presumed to have genetic mutations which lead to myocardial damage on a cellular level, but exactly which cellular changes that occur is still unknown.4, 5 In this stage, the heart is electrically and morphologically normal.6, 7, 8, 9 Dogs in the occult stage (stage 2) have either ventricular premature complexes (VPC) or a systolic dysfunction, or both, in the absence of overt clinical signs. Dogs in stage 3 have congestive heart failure (CHF) and overt clinical signs. Dogs with exercise intolerance or syncope due to arrhythmias are also in this group.6, 10, 11 Diagnosis of occult DCM in DP is based on echocardiography, 24‐hour ECG, and cardiac biomarkers.6, 12, 13, 14, 15, 16 However, dogs in stage 1 cannot be diagnosed by the gold standard methods, echocardiography and 24‐hour ECG. Cardiac troponin I (cTnI) concentration measurement is a valuable additional screening test for diagnosis at this early stage using a conventional cTnI assay. Using this conventional cTnI assay, a cutoff value of >0.22 ng/mL has a sensitivity of 79.5% and a specificity of 84.4% to detect all DCM disease stages.14
Cardiac troponin I is a highly sensitive and specific marker of myocardial injury and correlates with the severity of myocardial damage.17, 18 The development of high‐sensitivity cTnI (hs‐cTnI) assays in human medicine allows to detect lower cTnI concentrations compared with conventional cTnI assays. Therefore, the detection of mild myocardial injury, as it occurs in the early stages of myocardial diseases, is now possible.19, 20 Furthermore, in veterinary medicine, studies showed the feasibility and suitability of hs‐cTnI assays for recognition of myocardial damage, too.21, 22, 23, 24, 25 However, there is currently only 1 abstract available, which used an hs‐cTnI to detect DCM in DP with DCM.26
Therefore, the objectives of this study were (1) to evaluate an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics, Eschborn, Germany) analytically and clinically as a screening test for early stages of DCM in DP; (2) to provide cutoff values to detect DP with DCM using an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics); and (3) to perform a test comparison between an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics, Eschborn, Germany).
2. MATERIALS AND METHODS
2.1. Animals
The study population comprised 341 privately owned purebred DP that were selected from a continuing prospective longitudinal study, according to the inclusion and exclusion criteria. Overall 524 examinations at different time points from 341 DP were included in the study.
2.2. Inclusion criteria
Doberman Pinschers were selected if they fulfilled 1 of the group criteria as described below. For healthy DP, at least 1 follow‐up examination after 1 year needed to be available.
2.3. Exclusion criteria
Dogs were excluded if they could not be precisely matched into 1 of the 6 groups. Therefore, DP with 50‐300 VPC/24 hours once a year or dogs with unclear borderline echocardiographic measurements were considered to be “equivocal” and were excluded from the study. Doberman Pinschers diagnosed with relevant systemic diseases, congenital or acquired heart diseases other than DCM, were excluded. Furthermore, DP with increased urea concentrations, creatinine concentrations, or both were excluded from the study to avoid falsely increased concentrations of cTnI.
2.4. Examinations
Medical history was gathered and a complete examination was performed both at first presentation and at every control examination. Healthy DP were examined at least once a year. Doberman Pinschers with an increased cTnI concentration (using a conventional cTnI assay; Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) or short coupling interval of VPC (despite having less than 50 VPC/24 hours) were rechecked every 6 months. Doberman Pinschers with DCM were rechecked more frequently.
Each examination included a general clinical examination, echocardiography, 5‐minute ECG, 24‐hour ECG, and measurement of cTnI‐, urea‐, and creatinine concentrations. Complete blood count analyses and biochemistry screens were conducted when the result of the general clinical examination was abnormal.
2.5. Echocardiography
Echocardiography was performed in right and left lateral recumbency without sedation using a 2.0/3.5 MHz transducer (Vivid 7 dimensions; General Electric Medical Systems, Waukesha, Wisconsin) according to official recommendations.27 The left ventricular end‐systolic volume (LVESV) and left ventricular end‐diastolic volume (LVEDV) normalized to body surface area (BSA) were measured using Simpson's method of disc (SMOD). Dilated cardiomyopathy was diagnosed when LVESV/BSA > 55 mL/m2, LVEDV/BSA > 95 mL/m2, or both measurements were above the cutoff values.28 Simpson's method of disc was used for detection of DCM because of its superiority to other echocardiographic measures of left ventricular systolic function (eg, M‐Mode28 and sphericity index29), which is in accordance with current European Society of Veterinary Cardiology guidelines for screening for DCM in DP.16
2.6. Twenty‐four‐hour ECG
For 24‐hour ECG, analysis 1 of 2 analysis systems (Custo tera; Arcon Systems GmbH, Starnberg, Germany; Amedtech ECGpro Holter software, EP 810 digital Recorder; Medizintechnik Aue GmbH, Aue, Germany) was used. Manual adjustments and accuracy verification of the arrhythmias recognized by the semiautomatic software were performed. Dogs with >300 VPC/24 hours or 2 subsequent examinations within a year showing between 50 and 300 VPC/24 hours were considered to have DCM.16, 30
2.7. Blood sampling and analysis
Venous blood samples were collected both in tubes containing ethylendiaminetetraacetic acid (EDTA) and in serum tubes. Samples in EDTA tubes were centrifuged immediately after collection (4°C; 3500g). Serum samples were centrifuged 15‐20 minutes after collection (25°C; 4000g). One serum aliquot was shipped on the day of the blood collection to an external laboratory (IDEXX Laboratories, Ludwigsburg, Germany) for the measurement of the cTnI concentration, using a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) with a detection limit of 0.2 ng/mL (measuring range 0.2‐180 ng/mL). Remaining serum and EDTA‐plasma samples were stored in batches at −80°C until being shipped on dry ice to the same external laboratory (IDEXX Laboratories) for the determination of the cTnI concentrations from serum and EDTA‐plasma by the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics). The hs‐cTnI assay is a second‐generation sandwich immunoassay using 3 different antibodies and direct chemiluminometry. The detection limit of this assay is 0.006 ng/mL (measuring range 0.006‐50 ng/mL). Urea and creatinine concentrations were measured in‐house (Cobas Integra 400 plus; Roche Diagnostics, Rotkreuz, Switzerland).
2.8. Allocation
Dogs were allocated into 1 of the 6 groups based on the following criteria.
Healthy group: Dogs had no clinical signs, a normal echocardiographic examination (LVESV/BSA < 55 mL/m2 and LVEDV/BSA < 95 mL/m2),28 and <50 VPC/24 hours.12, 16, 31
Last‐normal group: At the time of the examination, dogs in this group were considered to be healthy as described for the healthy group. Dogs were assigned to the last‐normal group retrospectively when they were diagnosed with DCM (as described above) at their following examination. The last normal examination had to be performed within 1.5 years before the development of DCM.
VPC group: Dogs in this group had >300 VPC/24 hours or 2 subsequent examinations within a year showing between 50 and 300 VPC/24 hours.16, 30 The echocardiographic examination was normal.
ECHO group: Dogs had LVESV/BSA > 55 mL/m2 LVEDV/BSA > 95 mL/m2, or both above the cutoff values.16, 28 The result of the 24‐hour ECG examination was normal (<50 VPC/24 hours).12, 16, 31
ECHO‐and‐VPC group: Dogs were assigned to this group if they met criteria for both the VPC group and the ECHO group.
CHF group: Dogs had acute or stable CHF and were echocardiographically abnormal (LVESV/BSA > 55 mL/m2, LVEDV/BSA > 95 mL/m2, and LA/Ao > 1.5).16, 28 Thoracic X‐ray examination (showing cardiac pulmonary edema in combination with an enlarged left atrium) performed at the current or an earlier examination was required as confirmation for CHF. Arrhythmias could be present or not.
2.9. Statistical analysis
Statistical analyses were performed using commercially available software programs (PASW Statistics, Version 18.0; IBM Corporation, Armonk, New York; MedCalc Statistical Software Version 11.5.0.0.; Ostend, Belgium). A probability of P < .05 was regarded as statistically significant. All data were tested for normality by using the Kolmogorov‐Smirnov test. Continuous variables were depicted descriptively as median (interquartile range) for nonnormally distributed variables or mean (±SD for normally distributed variables. Comparison of dog characteristics was performed using an anova with a Bonferroni post hoc test. The test‐precision of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) was determined by calculating intra‐ and inter‐assay coefficient of variation (CV). The selection of serum samples for evaluation of test precision was randomly performed from the study population. Intra‐assay CV calculation resulted from 10‐fold analysis of 2 serum samples containing different cTnI concentrations. For determination of the inter‐assay CV, hs‐cTnI concentration measurement of 3 different serum samples was conducted once every 10 consecutive days. Samples were analyzed in duplicates, and the mean was used for further analysis. To assess conformity between cTnI concentrations obtained from serum and EDTA tubes using the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics), Bland‐Altman plot and Mann‐Whitney U test were used. These tests were also used for a comparison between cTnI concentrations obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics). The relationship between age and cTnI concentration was evaluated using a linear regression analysis in healthy DP. Mean cTnI concentrations of each group were calculated and concentrations were compared among the different groups using 1‐way ANOVA and Tamhane's T2 test. Cutoff values for detection of DP with DCM were determined using receiver operator curves (ROC) and the area under the curve (AUC). To assess the ability of these cutoff values to predict echocardiographic changes (ECHO group and ECHO‐and‐VPC group) or arrhythmias (VPC group and ECHO‐and‐VPC group), sensitivity, specificity, and AUC were calculated. A comparison of the ROC curves obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) was performed. The association between the number of VPC in 24 hours and the cTnI concentration was tested using a linear regression analysis. Furthermore, a stepwise multiple regression analysis was conducted, comparing the effect of age, sex, and disease stage on cTnI concentration of all groups. Receiver operator curve analysis was repeated after exclusion of DP with CHF.
3. RESULTS
3.1. Dog characteristics
Overall, 524 examinations of 341 DP (170 female and 171 male) met the inclusion criteria. The healthy group consisted of 179 DP, and results of the latest examination were used for statistical analyses in this group. One hundred sixty‐two DP (69 female and 93 male) were diagnosed with DCM, and results of each available examination (345 examinations) were used for statistical analyses. Table 1 shows characteristics of groups, results of echocardiographic measurements, and the number of VPC in 24 hours.
Table 1.
Study population characteristics, echocardiographic values, and number of VPC in 24 hours of healthy Doberman Pinschers (DP) and DP in 1 of 5 dilated cardiomyopathy groups (DCM)
| Healthy group | DP with DCM | |||||
|---|---|---|---|---|---|---|
| Last‐normal group | VPC group | ECHO group | ECHO‐and‐VPC group | CHF group | ||
| DP (n) | 179 | 29 | 45 | 24 | 41 | 23 |
| Examinations (n) | 179 | 29 | 119 | 61 | 100 | 36 |
| Female (n) | 101 | 11 | 29 | 9 | 13 | 7 |
| Male (n) | 78 | 18 | 15 | 15 | 29 | 16 |
| Age (y), mean ± SD | 7.5 ± 2.3 | 6.3 ± 2.2 | 8.3 ± 2.5 | 6.1 ± 2.3 | 8.2 ± 2.4 | 7.5 ± 2.2 |
| Body weight (kg), mean ± SD | 35.2 ± 5.2 | 37.9 ± 5.8 | 35.1 ± 5.1 | 35.6 ± 5.8 | 37.2 ± 5.5 | 36.1 ± 5.8 |
| LVEDV/BSA (mL/m2), mean ± SD | 74.2 ± 9.8 | 76.6 ± 8.8 | 79.1 ± 10.6 | 114.2 ± 21.3 | 114.8 ± 16.6 | 159.3 ± 57.9 |
| LVESV/BSA (mL/m2), mean ± SD | 37.8 ± 6.9 | 40.1 ± 7.9 | 41.2 ± 7.5 | 73.5 ± 21.4 | 72.3 ± 15.5 | 111.2 ± 46.4 |
| LA/Ao, mean ± SD | 1.3 ± 0.13 | 1.3 ± 0.16 | 1.3 ± 0.17 | 1.4 ± 0.34 | 1.4 ± 0.18 | 2.1 ± 0.41 |
| VPC/24 h (n), median (IQR) | 4 (1‐9) | 6 (2‐18) | 1018 (272‐3696) | 11 (3‐18) | 1371.5 (320‐5426) | 318 (128‐4317) |
Abbreviations: CHF, congestive heart failure; ECHO, echocardiography; IQR, interquartile range; LA/Ao, short‐axis ratio of diastolic left atrial diameter to aortic root diameter; LVEDV/BSA and LVESV/BSA, left ventricular end‐systolic volume and end‐diastolic volume normalized to body surface area; VPC, ventricular premature complexes.
Doberman Pinschers in the VPC group were significantly older than dogs in the healthy group (P < .001), ECHO group (P < .001), and last‐normal group (P < .001). Dogs in the ECHO group were significantly younger than dogs in the healthy group (P < .001), VPC group (P < .001), and ECHO‐and‐VPC group (P < .001). Furthermore, dogs in the last‐normal group were significantly younger than dogs in the ECHO‐and‐VPC group (P < .001).
3.2. Analytical evaluation of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and analytical test comparison between the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics)
The mean intra‐assay CV of the hs‐cTnI assay was 2.8% and the mean inter‐assay CV was 5.5% (Table 2). To assess the conformity between hs‐cTnI concentrations obtained from serum and EDTA tubes, 128 paired blood samples of 64 DP (64 serum samples, 64 EDTA samples) were available. At the beginning of the study, these 64 DP were randomly selected and for these dogs, both serum and plasma samples were available. High‐sensitivity cTnI concentration was measured in each sample using the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and resulted in a mean serum cTnI concentration of 0.29 ng/mL and a mean EDTA‐plasma cTnI concentration of 0.26 ng/mL (mean difference: 0.03 ng/mL [14.4%]; SD of the difference: ± 0.05 ng/mL; 95% confidence interval (CI0.95) = 0.01‐0.04 ng/mL; lower limit of agreement: −0.07 ng/mL; upper limit of agreement: 0.13 ng/mL) (Figure 1). There was no statistically significant difference between the mean serum and EDTA‐plasma cTnI concentrations (P = .47).
Table 2.
Mean (ng/mL), SD, and CV (%) of hs‐cTnI concentration measurement for evaluation of intra‐ and inter‐assay precision
| Serum sample | Mean | SD | CV |
|---|---|---|---|
| Intra‐assay precision | |||
| 1 | 0.98 | 0.02 | 2.3 |
| 2 | 0.24 | 0.01 | 3.3 |
| Inter‐assay precision | |||
| 3 | 0.94 | 0.05 | 5.8 |
| 4 | 0.22 | 0.01 | 5.2 |
| 5 | 0.17 | 0.01 | 5.6 |
Abbreviations: CHF, congestive heart failure; CTnI, cardiac troponin I; CV, coefficient of variation; hs‐cTnI, high‐sensitivity cTnI; VPC, ventricular premature complexes.
Figure 1.
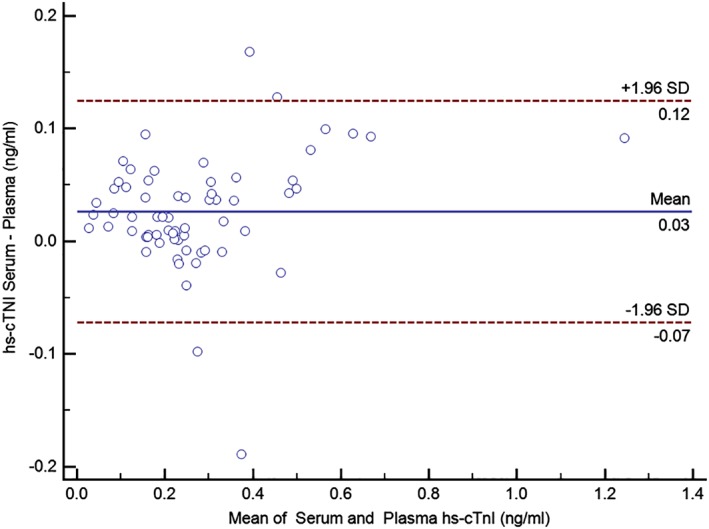
This Bland‐Altman plot displays the agreement between cTnI concentrations obtained from serum and EDTA‐plasma using an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics). CTnI, cardiac troponin I; EDTA, ethylendiaminetetraacetic acid; hs‐cTnI, high‐sensitivity cTnI
For an analytical comparison between cTnI concentration obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics), cTnI concentrations below the detection limit of the assays were excluded from this analysis. Accordingly, 372 blood samples were available for the comparison. The following mean concentrations of cTnI were detected: hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) using EDTA‐plasma: 0.26 ng/mL and conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) using serum: 0.49 ng/mL. There was a mean difference of 0.22 ng/mL (64.5%), SD of the difference ± 0.15 ng/mL, CI0.95 = 0.21‐0.24 ng/mL, lower limit of agreement: −0.08 ng/mL, and upper limit of agreement: 0.52 ng/mL (Figure 2). The difference between cTnI concentrations obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) was statistically significant (P < .0001).
Figure 2.
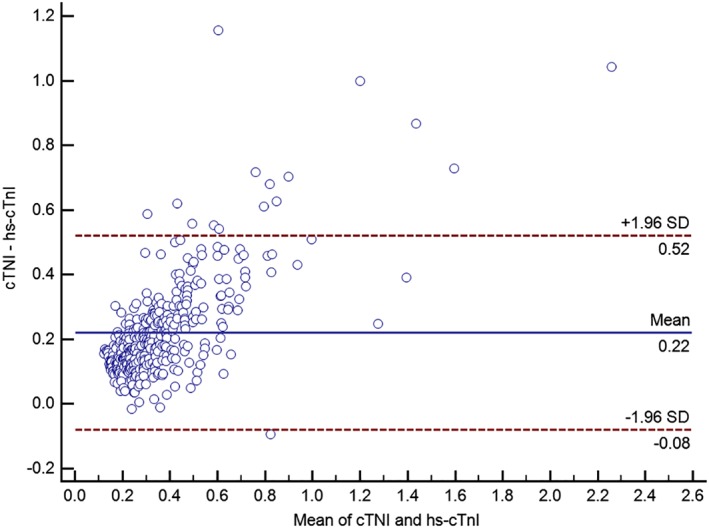
Bland‐Altman plot for displaying the agreement between cTnI concentrations obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) (using EDTA‐plasma) and a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) (using serum). CTnI, cardiac troponin I; EDTA, ethylendiaminetetraacetic acid; hs‐cTnI, high‐sensitivity cTnI
3.3. Clinical evaluation of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) using EDTA‐plasma
The relationship between age and cTnI concentration was evaluated using a linear regression analysis in 179 healthy DP (Figure 3). Despite only a weak association between age and cTnI concentration (R2 = 0.095), older dogs had significantly higher EDTA‐plasma hs‐cTnI concentrations (P < .001). There was no difference between the hs‐cTnI concentration from healthy male (mean = 0.095 ng/mL) and female (mean = 0.087 ng/mL) dogs (P = .91).
Figure 3.
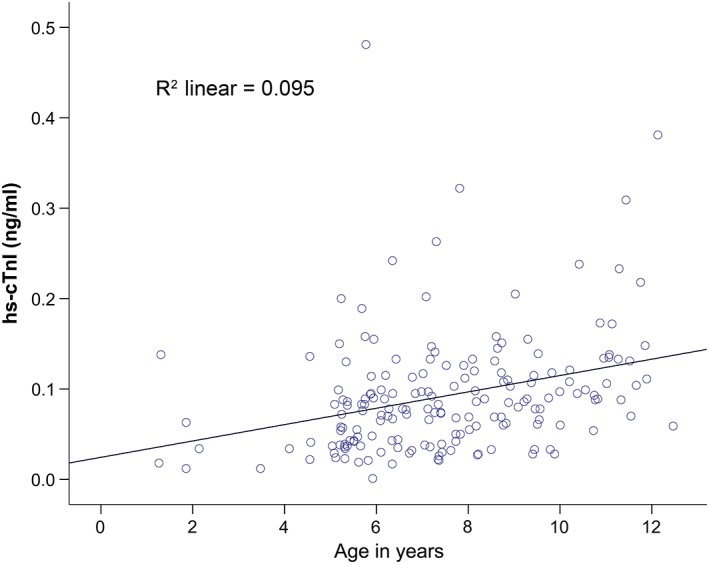
Relationship between age and EDTA‐plasma cTnI concentration (ng/mL) in healthy Doberman Pinschers using an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) (P < .001). CTnI, cardiac troponin I; EDTA, ethylendiaminetetraacetic acid; hs‐cTnI, high‐sensitivity cTnI
Table 3 shows EDTA‐plasma hs‐cTnI concentrations of each group. The following statistically significant differences between the mean hs‐cTnI concentrations of these groups were detected: DP in the healthy group had a lower mean hs‐cTnI concentration than DP in all other groups (P < .001), including the last‐normal group. DP in the ECHO‐and‐VPC group had a higher mean cTnI concentration than dogs in the ECHO group (P < .001), last‐normal group (P = .002), and healthy group (P < .001). Furthermore, dogs in the CHF group had a significantly higher mean cTnI concentration than dogs in all other groups (P < .001) (Figure 4).
Table 3.
High‐sensitivity EDTA‐plasma cardiac troponin I concentration (ng/mL) in different groups of Doberman Pinschers
| N | Mean ± SD | |
|---|---|---|
| Healthy group | 179 | 0.092 ± 0.066 |
| Last‐normal group | 29 | 0.168 ± 0.091 |
| VPC group | 119 | 0.211 ± 0.130 |
| ECHO group | 61 | 0.173 ± 0.135 |
| ECHO‐and‐VPC group | 100 | 0.296 ± 0.277 |
| CHF group | 36 | 0.593 ± 0.479 |
Abbreviations: CHF, congestive heart failure; ECHO, echocardiography; EDTA, ethylendiaminetetraacetic acid; VPC, ventricular premature complexes.
Figure 4.
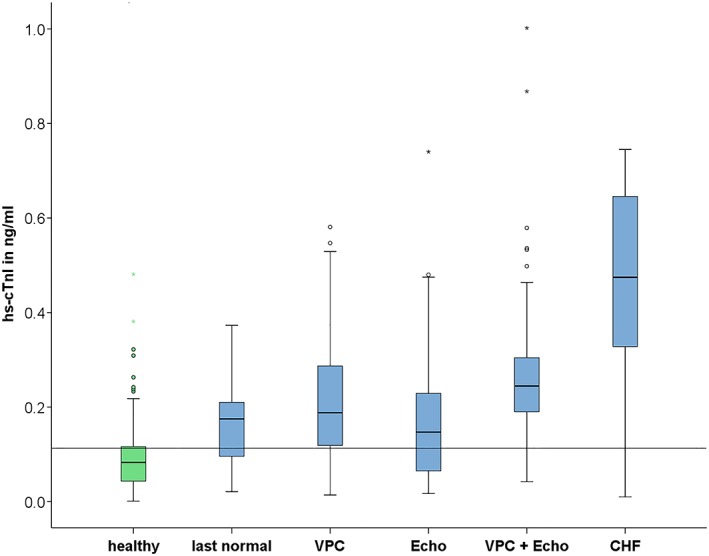
Box plots for graphically depicting the distribution of 524 EDTA‐plasma hs‐cTnI concentrations of 179 healthy Doberman Pinschers and 162 Doberman Pinschers with DCM in different disease groups. Each plot displays the interquartile range (IQR) and the band inside the box is the median. The whiskers are 1.5 times the IQR. Circles and asterisks represent outlier concentrations 1.5‐3 times and >3 times the IQR, respectively. The horizontal line displays the cutoff value of hs‐cTnI concentration >0.113 ng/mL. CHF, congestive heart failure; CTnI, cardiac troponin I; DCM, dilated cardiomyopathy; EDTA, ethylendiaminetetraacetic acid; hs‐CTnI, high‐sensitivity cTnI; VPC, ventricular premature complexes
3.4. Cutoff values for detection of DP with DCM using an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and EDTA‐plasma
Several cutoff values for overall DCM diagnosis and for subgroups were tested by using ROC curves. With a cutoff value of hs‐cTnI concentration >0.113 ng/mL, sensitivity was 81.2%, specificity was 73.2% and AUC was 0.835 for detection of DCM in DP. The prevalence of DCM in the current population was 65.8%. Concerning DP in the last‐normal group, 72% (21/29) of these dogs were presumed to be affected by DCM when using an hs‐cTnI concentration >0.113 ng/mL as cutoff value. With a higher cutoff value of hs‐cTnI concentration >0.242 ng/mL to identify all DCM stages, including dogs in the last‐normal group, it had increased specificity but only a low sensitivity (sensitivity 42.3%, specificity 97.2%). The AUC for prediction of VPC (AUC = 0.862) was similar to the AUC for prediction of echocardiographic changes (AUC = 0.838). Sensitivity, specificity, and AUC of hs‐cTnI concentration cutoff values to predict DCM, only echocardiographic changes, or only arrhythmias in DP are shown in Table 4. There was no statistically significant association between the hs‐cTnI concentration and the number of VPC in 24 hours (R2 = 0.013; P = .22) for DP with arrhythmias. The multiple regression analysis showed a statistically significant influence (P < .001) of disease stage and age on cTnI concentration. The influence of sex was statistically not significant.
Table 4.
Sensitivity, specificity, and AUC of cTnI concentration cutoff values to predict dilated cardiomyopathy (DCM), only echocardiographic changes or only arrhythmias in Doberman Pinschers using an hs‐cTnI (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) assay and a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics)
| Assay | Cutoff value | All DCM groups | VPC group | Echo group | |
|---|---|---|---|---|---|
| Prevalence (%) | 65.8 | 55.0 | 47.4 | ||
| Hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) | >0.113 ng/mL | Sensitivity (%) | 81.2 | 85.4 | 82.0 |
| Specificity (%) | 73.2 | 73.2 | 73.2 | ||
| AUC | 0.835 | 0.862 | 0.838 | ||
| >0.242 ng/mL | Sensitivity (%) | 42.3 | 42.9 | 40.4 | |
| Specificity (%) | 97.2 | 97.2 | 97.2 | ||
| AUC | 0.835 | 0.862 | 0.838 | ||
| Conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) | >0.22 ng/mL | Sensitivity (%) | 80.5 | 83.5 | 81.7 |
| Specificity (%) | 72.4 | 72.4 | 72.4 | ||
| AUC | 0.813 | 0.826 | 0.814 |
Abbreviations: AUC, area under the curve; cTnI, cardiac troponin I; hs‐cTnI, high‐sensitivity cTnI; VPC, ventricular premature complexes.
3.5. Clinical evaluation of the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) using serum samples
The optimal cutoff value was the same as previously published >0.22 ng/mL14 (conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics)) in this study population and revealed a sensitivity of 80.5%, a specificity of 72.4%, and AUC of 0.813 to detect all stages of DCM. With this cutoff value of 62% (18/29), DP in the last‐normal group were presumed to be affected by DCM. The AUC, sensitivity, and specificity for prediction of DCM, arrhythmias, or echocardiographic changes are displayed in Table 4.
A comparison of the ROC curves obtained from the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) showed no statistically significant difference (P = .07).
3.6. Analysis after exclusion of Doberman Pinschers with CHF
After exclusion of DP with CHF, the AUC of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) (0.828) was significantly (P = .02) larger compared to the AUC of the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) (0.790) to detect occult cardiomyopathy in DP (Figure 5). With a cutoff value of >0.113 ng/mL (hs‐cTnI assay; Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics), sensitivity was 79.3% and specificity was 73.2%. With a cutoff value of >0.22 ng/mL14 (conventional cTnI assay, Immulite 2000 troponin I test; Siemens Healthcare Diagnostics), sensitivity was 78.3% and specificity was 71.9%.
Figure 5.
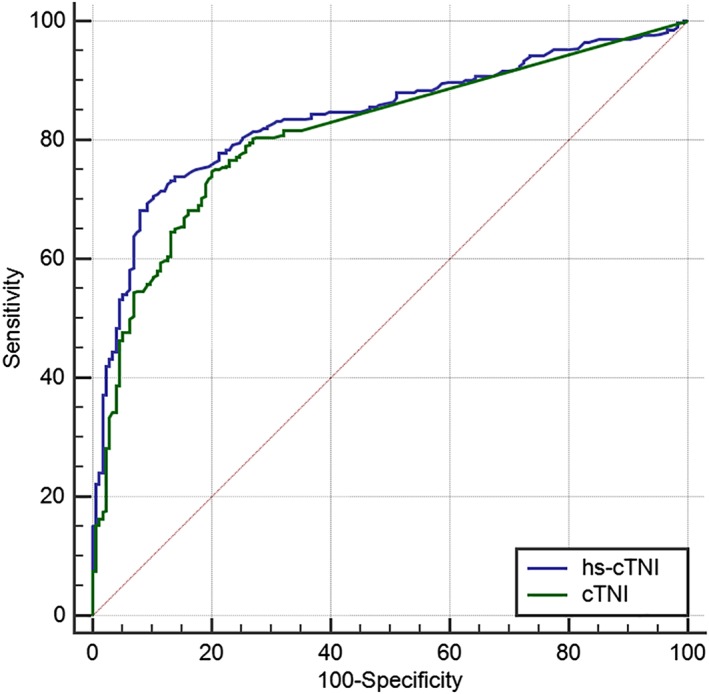
Comparison of ROC curves obtained from an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) (using EDTA‐plasma) and a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) (using serum) to detect occult DCM in Doberman Pinschers. CTnI, cardiac troponin I; EDTA, ethylendiaminetetraacetic acid; hs‐cTnI, high‐sensitivity cTnI; ROC, receiver operator curves
4. DISCUSSION
Cardiac troponin I is an important marker of myocardial damage. This prospective study evaluated an hs‐cTnI assay analytically and clinically as a screening test for various stages of DCM in DP and provided cutoff values for detection of DP with DCM. With a cutoff value of hs‐cTnI concentration of >0.113 ng/mL, the presence of DCM in DP was presumed with a good sensitivity. Therefore, in combination with the gold standard examinations, echocardiography and 24‐hour ECG, this hs‐cTnI cutoff value might be used for DCM screening purposes. With higher cutoff values, for example, hs‐cTnI concentration >0.242 ng/mL, the specificity to detect all DCM disease stages increased. If DP have an hs‐cTnI concentration above this cutoff value, the high specificity indicates that the dogs have DCM with a high probability. However, it is important to acknowledge that cTnI can increase with a variety of noncardiac diseases, such as gastric dilatation volvulus,32 canine babesiosis,33 kidney diseases,34 pulmonary hypertension,35 and systemic inflammatory response syndrome.36 In our study, the included dogs did not have clinical signs, with the exception of the clinical group, and there was no evidence of pulmonary hypertension detectable on echocardiography. Therefore, it is recommended to use the suggested cutoff values for screening purposes only in clinically normal dogs, without evidence of a systemic disease. Accordingly, statistical ROC analysis was repeated after exclusion of DP with CHF (with clinical signs) and results showed the same specificity and a similar but a slightly lower sensitivity for detection of DP with occult DCM.
Gold standard examinations for the diagnosis of DCM are a combination of echocardiography and 24‐hour ECG. In this study, DP in the last‐normal group showed a normal echocardiographic and 24‐hour ECG examination. Therefore, at the time of the first examination, dogs in this group were considered to be healthy according to the gold standard examinations. These dogs were included into the last‐normal group retrospectively when they were diagnosed with DCM on the following examination.
The optimal cutoff value for the conventional cTnI test (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) was the same as previously published (>0.22 ng/mL14) for detection of DP with DCM and showed a similar sensitivity but a slightly lower specificity in this study population in comparison to the cited study.14 Therefore, this study confirms the previously published cutoff value of cTnI concentration >0.22 ng/mL using a conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics).
The comparison between the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) and conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) indicated a similar good test performance for prediction of DCM in DP (Figure 5 and Table 4). Nevertheless, a better detection rate of DP in the last‐normal group (DP with DCM stage 1) was reached using the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics). Accordingly, more dogs with DCM stage 1 could be identified using an hs‐cTnI assay, indicating less advanced disease. This advantage might be attributed to the lower detection limit of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics). Earlier detection of DCM in DP by using cTnI concentration measurement in comparison to echocardiography and 24‐hour ECG corroborates the findings of other studies in veterinary14 and human37 medicine.
This prospective study showed that the detection of mild myocardial injury in DP, as it occurs in early stages of DCM, is possible and feasible, by using an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) or alternatively by using the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics). However, the comparison between the conventional (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) and the hs‐cTnI (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) assay showed that the conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) measures significantly higher values compared to the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics). This is graphically depicted on the Bland‐Altman plot (Figure 2), which shows a random difference between the 2 assays, and it therefore illustrates that the 2 assays cannot be used interchangeably. Accordingly, for follow‐up and diagnostic purposes, it is recommended to use the same assay and different cutoff values.
Currently, only a few studies evaluated the suitability of an hs‐cTnI assay for detection of early myocardial damage in animals,21, 22, 23, 24, 25 and there is only 1 study published as an abstract evaluating an hs‐cTnI assay in DP.26 This study in 449 DP reported an hs‐cTnI cutoff >0.139 ng/mL (ADVIA Centaur CP Ultra‐TnI) and had a 100% sensitivity and 79% specificity to detect the characteristic echocardiographic morphologic changes of DCM with or without concurrent evidence of VPC on a 3‐minute ECG.26 The calculated cutoff value (>0.139 ng/mL) of this study is very similar to the cutoff value of our study (>0.113 ng/mL) but had a better sensitivity and specificity than in our study. The difference between the results might be explained by the low number of dogs with DCM (n = 22) in the cited study, presumably leading to a higher variation. Furthermore, no Holter examination was performed and no SMOD was used for detection of DP with DCM in the cited study. Because SMOD is better for detection of DCM in DP in comparison to M‐mode measurements28 and Holter examination is necessary for diagnosis of DCM16, this study (and the lower hs‐cTnI cutoff value) potentially detected more DP with occult DCM in comparison to the cited study.
Nevertheless, using hs‐cTnI assays might lead to an earlier detection of DCM in DP, and dogs with increased hs‐cTnI concentrations could benefit from earlier control examinations (such as recheck after 6 months) compared to the recommendation of yearly reexaminations.16 In a similar manner, in human medicine, the application of hs‐cTnI assays is associated with a major progress in early recognition of patients with acute coronary syndrome38 and helps defining which patient would benefit from early invasive management.39, 40, 41, 42
The international recommendations43, 44 for test precision (CV <10%) were fulfilled, and inter‐assay variability as well as intra‐assay variability revealed similar results as described previously for cattle, cats, and dogs with different cardiac diseases.21, 23, 25 The proposed hs‐cTnI cutoff concentration 0.113 ng/mL is lower than the concentration of the samples used for validation, and the majority of dogs in this study had hs‐cTnI concentrations lower than the samples used for validation. However, the hs‐cTnI (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) assay has been evaluated in a previous study using high and low cTnI serum pools with intra‐ and inter‐assay CVs ranging from 3.9% to 6.4% and from 2.7% to 4.7%, respectively.21 Therefore, the samples used for validation of the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) in the present study should be seen as supplements to the cited study.21
The assessment of the conformity between hs‐cTnI concentrations obtained from serum and EDTA tubes showed a mean difference of 0.03 ng/mL, which was statistically not significant. Therefore, serum as well as EDTA blood can be used for hs‐cTnI measurement. Some authors described that ionized calcium, which is required to maintain cTnI complexes, is removed by chelation of metal ions, when using EDTA tubes.45 This might be a cause for the detection of lower cTnI concentration in EDTA tubes.
In this study, the AUC for prediction of arrhythmias was similar to the AUC for prediction of echocardiographic changes. It is presumed that myocardial damage is more relevant in connection with arrhythmias than in connection with echocardiographic changes.46 However, results of another study suggested a better prediction of echocardiographic changes in contrast to the prediction of VPC and contradicts this presumption.14 Such inconsistency might be explained by the different number of dogs in the respective studies. Nevertheless, the nonexistent association between cTnI concentration and the number of VPC in 24 hours conforms with the same previous study, investigating cTnI concentrations in DP.14
Despite earlier detection of DCM compared to gold standard examinations in some dogs, hs‐cTnI concentration measurement should not and cannot replace echocardiography and 24‐hour ECG examinations. For assessment of the malignancy of VPC (including fastest rate of VPC, presence of ventricular tachycardia, couplets, and triplets), a 24‐hour ECG is required.47 The initiation of treatment of systolic dysfunction is based on echocardiography.48 However, cTnI concentration measurement is a simple, feasible, low‐cost, and useful screening test for DCM in DP. Doberman Pinschers with hs‐cTnI concentrations >0.113 ng/mL should be investigated using gold standard examinations (echocardiography and 24‐hour ECG). Furthermore, DP with a negative assay result (hs‐cTnI concentration <0.113 ng/mL) should be examined using gold standard examinations as well, not to miss DCM.
In this study, age had a statistically significant influence on healthy DP, that is, older dogs showed higher hs‐cTnI concentrations. This is in accordance with other studies suggesting that an age‐related remodeling of the myocardium leads to increased cTnI concentrations.14, 17, 46 There was no influence of sex on hs‐cTnI concentration in this study population, as likewise described in another study.14
4.1. Study limitations
It cannot be ruled out that healthy dogs (according to gold standard examinations) with an increased cTnI concentration were in an early stage of DCM, which presents a limitation of this study. It is possible that some of these dogs might develop DCM or that the concentrations were increased due to (undetected) systemic diseases. A follow‐up study of these dogs would be interesting, as it might potentially lower the calculated cutoff values. Furthermore, there are several extra cardiac diseases that can cause an increased cTnI concentration. Therefore, a medical history and general clinical examination was performed at each examination. Blood checks were conducted when the result of the general clinical examination was abnormal. Nevertheless, it cannot be completely ruled out that dogs with an undetected extra cardiac disease were part of the study population and they might potentially have influenced the results. In addition, because examinations of the last‐normal group were performed up to 1.5 years previous of a DCM diagnosis, it is uncertain at what point between these examinations individual dogs started to have cellular changes. Furthermore, cellular changes are assumed based on cTnI concentrations but have not been verified by biopsies (for obvious reasons in privately owned dogs), which also presents a limitation of this study.
5. CONCLUSION
A cutoff value of hs‐cTnI concentration >0.113 ng/mL might be the first indicator of the presence of DCM in DP and should be further evaluated by echocardiography and 24‐hour‐ECG. The conventional cTnI assay (Immulite 2000 troponin I test; Siemens Healthcare Diagnostics) performed similarly well as the hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics), but detected about 10% less dogs in the last‐normal stage. Application of an hs‐cTnI assay (Advia Centaur TnI‐Ultra assay; Siemens Healthcare Diagnostics) might lead to identification of DP with DCM.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study complies with the regulations of the German Animal Welfare Act and owners provided written consent to participate in the study. No 60‐November 18, 2015, LMU Ethic commission.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Work was done at the Clinic of Small Animal Medicine, LMU University Munich.
Klüser L, Maier ET, Wess G. Evaluation of a high‐sensitivity cardiac troponin I assay compared to a first‐generation cardiac troponin I assay in Doberman Pinschers with and without dilated cardiomyopathy. J Vet Intern Med. 2019;33:54–63. 10.1111/jvim.15384
REFERENCES
- 1. Wess G, Schulze A, Butz V, et al. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med. 2010;24:533‐538. [DOI] [PubMed] [Google Scholar]
- 2. Calvert CA. Dilated congestive cardiomyopathy in doberman pinschers. Comp Cont Educ Pract. 1986;8:417‐430. [Google Scholar]
- 3. Meurs KM, Fox PR, Norgard M, et al. A prospective genetic evaluation of familial dilated cardiomyopathy in the Doberman pinscher. J Vet Intern Med. 2007;21:1016‐1020. [DOI] [PubMed] [Google Scholar]
- 4. Mausberg TB, Wess G, Simak J, et al. A locus on chromosome 5 is associated with dilated cardiomyopathy in Doberman pinschers. PLoS One. 2011;6:e20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owczarek‐Lipska M, Mausberg TB, Stephenson H, Dukes‐McEwan J, Wess G, Leeb T. A 16‐bp deletion in the canine PDK4 gene is not associated with dilated cardiomyopathy in a European cohort of Doberman pinschers. Anim Genet. 2013;44:239. [DOI] [PubMed] [Google Scholar]
- 6. O'Grady MR, O'Sullivan ML. Dilated cardiomyopathy: an update. Vet Clin Small Anim Pract. 2004;34:1187‐1207. [DOI] [PubMed] [Google Scholar]
- 7. Calvert CA, Meurs KM. Cardiomyopathy in Doberman pinschers In: Bonagura JD, Twedt DC, eds. Current Veterinary Therapy. St. Louis: Saunders Elsevier; 2009:800‐803. [Google Scholar]
- 8. McCutcheon LJ, Cory CR, Nowack L, et al. Respiratory chain defect of myocardial mitochondria in idiopathic dilated cardiomyopathy of Doberman pinscher dogs. Can J Physiol Pharmacol. 1992;70:1529‐1533. [DOI] [PubMed] [Google Scholar]
- 9. Lopes R, Solter PF, Sisson DD, Oyama MA, Prosek R. Characterization of canine mitochondrial protein expression in natural and induced forms of idiopathic dilated cardiomyopathy. Am J Vet Res. 2006;67:963‐970. [DOI] [PubMed] [Google Scholar]
- 10. Calvert CA, Hall G, Jacobs G, Pickus C. Clinical and pathologic findings in Doberman pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984‐1991). J Am Vet Med Assoc. 1997;210:505‐511. [PubMed] [Google Scholar]
- 11. Calvert CA, Chapman WL Jr, Toal RL. Congestive cardiomyopathy in Doberman pinscher dogs. J Am Vet Med Assoc. 1982;181:598‐602. [PubMed] [Google Scholar]
- 12. Calvert CA, Jacobs G, Pickus CW, Smith DD. Results of ambulatory electrocardiography in overtly healthy Doberman Pinschers with echocardiographic abnormalities. J Am Vet Med Assoc. 2000;217:1328‐1332. [DOI] [PubMed] [Google Scholar]
- 13. Singletary GE, Morris NA, Lynne O'Sullivan M, Gordon SG, Oyama MA. Prospective evaluation of NT‐proBNP assay to detect occult dilated cardiomyopathy and predict survival in Doberman Pinschers. J Vet Intern Med. 2012;26:1330‐1336. [DOI] [PubMed] [Google Scholar]
- 14. Wess G, Simak J, Mahling M, Hartmann K. Cardiac troponin I in Doberman Pinschers with cardiomyopathy. J Vet Intern Med. 2010;24:843‐849. [DOI] [PubMed] [Google Scholar]
- 15. Wess G, Butz V, Mahling M, Hartmann K. Evaluation of N‐terminal pro‐B‐type natriuretic peptide as a diagnostic marker of various stages of cardiomyopathy in Doberman pinschers. Am J Vet Res. 2011;72:642‐649. [DOI] [PubMed] [Google Scholar]
- 16. Wess G, Domenech O, Dukes‐McEwan J, Häggström J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J Vet Cardiol. 2017;19:405‐415. [DOI] [PubMed] [Google Scholar]
- 17. Oyama MA, Sisson DD. Cardiac troponin‐I concentration in dogs with cardiac disease. J Vet Intern Med. 2004;18:831‐839. [DOI] [PubMed] [Google Scholar]
- 18. Spratt DP, Mellanby RJ, Drury N, Archer J. Cardiac troponin I: evaluation I of a biomarker for the diagnosis of heart disease in the dog. J Small Anim Pract. 2005;46:139‐145. [DOI] [PubMed] [Google Scholar]
- 19. Schultze AE, Konrad RJ, Credille KM, Lu QA, Todd J. Ultrasensitive cross‐species measurement of cardiac troponin‐I using the Erenna immunoassay system. Toxicol Pathol. 2008;36:777‐782. [DOI] [PubMed] [Google Scholar]
- 20. Tate JR. Troponin revisited 2008: assay performance. Clin Chem Lab Med. 2008;46:1489‐1500. [DOI] [PubMed] [Google Scholar]
- 21. Langhorn R, Willesen JL, Tarnow I, Kjelgaard‐Hansen M. Evaluation of a high‐sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet Clin Pathol. 2013;42:490‐498. [DOI] [PubMed] [Google Scholar]
- 22. Serra M, Papakonstantinou S, Adamcova M, O'Brien PJ. Veterinary and toxicological applications for the detection of cardiac injury using cardiac troponin. Vet J. 2010;185:50‐57. [DOI] [PubMed] [Google Scholar]
- 23. Winter RL, Saunders AB, Gordon SG, et al. Analytical validation and clinical evaluation of a commercially available high‐sensitivity immunoassay for the measurement of troponin I in humans for use in dogs. J Vet Cardiol. 2014;16:81‐89. [DOI] [PubMed] [Google Scholar]
- 24. Apple FS, Murakami MM, Ler R, Walker D, York M, for the HESI Technical Committee of Biomarkers Working Group on Cardiac Troponins . Analytical characteristics of commercial cardiac troponin I and T immunoassays in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clin Chem. 2008;54:1982‐1989. [DOI] [PubMed] [Google Scholar]
- 25. Varga A, Schober KE, Walker WL, Lakritz J, Michael Rings D. Validation of a commercially available immunoassay for the measurement of bovine cardiac troponin I. J Vet Intern Med. 2009;23:359‐365. [DOI] [PubMed] [Google Scholar]
- 26. Gordon SG, Estrada AH, Braz‐Ruivo L, et al. Evaluation of NTproBNP, high sensitivity troponin I and PDK4 for the detection of occult DCM: a prospective study in 449 Doberman Pinschers. (Oral Abstract ECVIM Forum 2015). J Vet Intern Med. 2016;30:365. [Google Scholar]
- 27. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 28. Wess G, Mäurer J, Simak J, Hartmann K. Use of Simpson's method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med. 2010;24:1069‐1076. [DOI] [PubMed] [Google Scholar]
- 29. Holler PJ, Wess G. Sphericity index and E‐point‐to‐septal‐separation (EPSS) to diagnose dilated cardiomyopathy in Doberman Pinschers. J Vet Intern Med. 2014;28:123‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geraghty N, Wess G. Vergleich verschiedener Holterkriterien zur Diagnose des arrhythmischen Stadiums der dilatativen Kardiomyopathie beim Dobermann Tierärztliche Fakultät der LMU München. Munich, Germany: LMU Veterinary Faculty; 2011:1‐107. [Google Scholar]
- 31. Calvert CA, Wall M. Results of ambulatory electrocardiography in overtly healthy Doberman Pinschers with equivocal echocardiographic evidence of dilated cardiomyopathy. J Am Vet Med Assoc. 2001;219:782‐784. [DOI] [PubMed] [Google Scholar]
- 32. Schober KE, Cornand C, Kirbach B, Aupperle H, Oechtering G. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation‐volvulus. J Am Vet Med Assoc. 2002;221:381‐388. [DOI] [PubMed] [Google Scholar]
- 33. Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med. 2002;16:63‐68. [DOI] [PubMed] [Google Scholar]
- 34. Porciello F, Rishniw M, Herndon WE, Birettoni F, Antognoni MT, Simpson KW. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J. 2008;86:390‐394. [DOI] [PubMed] [Google Scholar]
- 35. Guglielmini C, Civitella C, Diana A, di Tommaso M, Cipone M, Luciani A. Serum cardiac troponin I concentration in dogs with precapillary and postcapillary pulmonary hypertension. J Vet Intern Med. 2010;24:145‐152. [DOI] [PubMed] [Google Scholar]
- 36. Hamacher L, Dorfelt R, Muller M, et al. Serum cardiac troponin I concentrations in dogs with systemic inflammatory response syndrome. J Vet Intern Med. 2015;29:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato Y, Kita T, Takatsu Y, Kimura T. Biochemical markers of myocyte injury in heart failure. Heart. 2004;90:1110‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melanson SE, Morrow DA, Jarolim P. Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol. 2007;128:282‐286. [DOI] [PubMed] [Google Scholar]
- 39. Venge P, Lagerqvist B, Diderholm E, Lindahl B, Wallentin L. Clinical performance of three cardiac troponin assays in patients with unstable coronary artery disease (a FRISC II substudy). Am J Cardiol. 2002;89:1035‐1041. [DOI] [PubMed] [Google Scholar]
- 40. Eggers KM, Oldgren J, Nordenskjold A, et al. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004;148:574‐581. [DOI] [PubMed] [Google Scholar]
- 41. Amodio G, Antonelli G, Varraso L, Ruggieri V, di Serio F. Clinical impact of the troponin 99th percentile cut‐off and clinical utility of myoglobin measurement in the early management of chest pain patients admitted to the emergency cardiology department. Coron Artery Dis. 2007;18:181‐186. [DOI] [PubMed] [Google Scholar]
- 42. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858‐867. [DOI] [PubMed] [Google Scholar]
- 43. Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AH. Quality specifications for cardiac troponin assays. Clin Chem Lab Med. 2001;39:175‐179. [DOI] [PubMed] [Google Scholar]
- 44. Panteghini M, Pagani F, Yeo KT, et al. Evaluation of imprecision for cardiac troponin assays at low‐range concentrations. Clin Chem. 2004;50:327‐332. [DOI] [PubMed] [Google Scholar]
- 45. Liao R, Wang CK, Cheung HC. Coupling of calcium to the interaction of troponin I with troponin C from cardiac muscle. Biochemistry. 1994;33:12729‐12734. [DOI] [PubMed] [Google Scholar]
- 46. Clerico A, Fortunato A, Ripoli A, Prontera C, Zucchelli GC, Emdin M. Distribution of plasma cardiac troponin I values in healthy subjects: pathophysiological considerations. Clin Chem Lab Med. 2008;46:804‐808. [DOI] [PubMed] [Google Scholar]
- 47. Kluser L, Holler PJ, Simak J, et al. Predictors of sudden cardiac death in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med. 2016;30:722‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med. 2012;26:1337‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]


