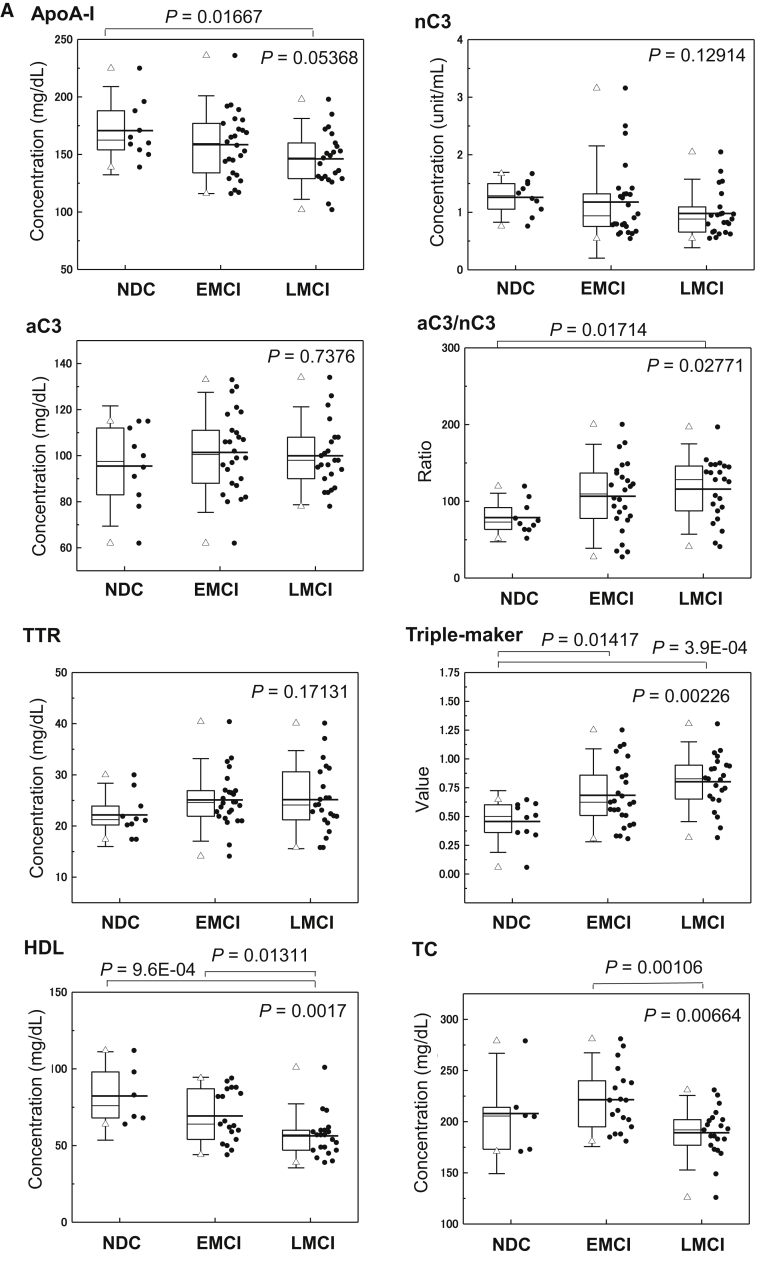
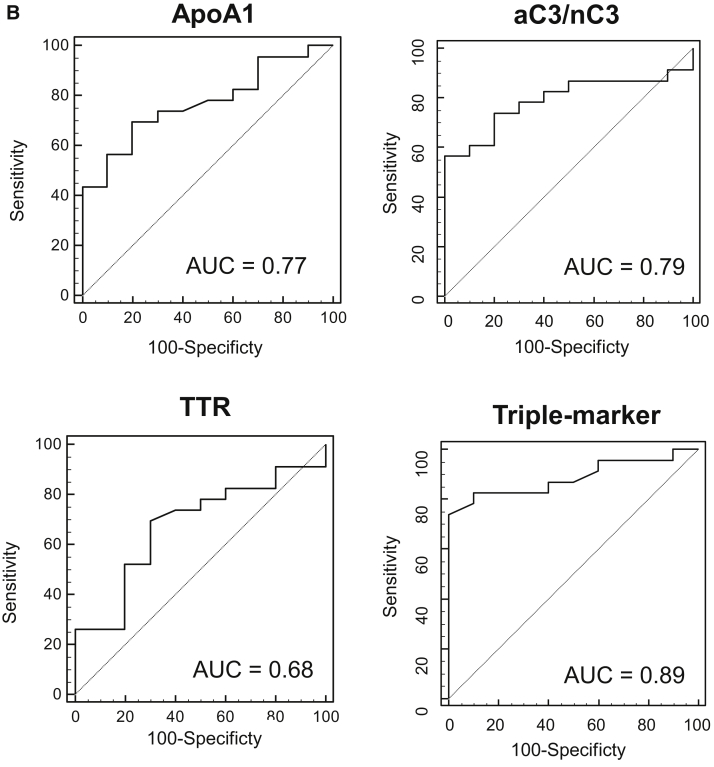
Fig. 1.
Clinical validity of Aβ clearance–associated proteins in the serum as blood-based biomarkers for EMCI and LMCI. (A) Serum levels of aC3, nC3, apoA-I, aC3/nC3, TTR, triple markers (apoA-I, aC3/nC3, and TTR), HDL, and TC in EMCI (n = 26), LMCI (n = 23), and NDC (n = 10) subjects. The bold solid bars within the boxplot represent the median abundance, and the solid bars represent the mean abundance for the given group. Open triangles are the highest and lowest values in each group. Error bars represent ±1.5 standard deviation. Statistical differences among the three groups (NDC, EMCI, and LMCI) were evaluated by the Kruskal-Wallis test. (B) C-statistics of sequester protein (apoA-I, aC3/nC3, TTR, and triple markers) levels demonstrated clinical potential in discriminating LMCI from NDC subjects. Abbreviations: aC3, active form of C3; nC3, native form of C3; TC, total cholesterol; TTR, transthyretin; NDC, nondemented disease control; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment.