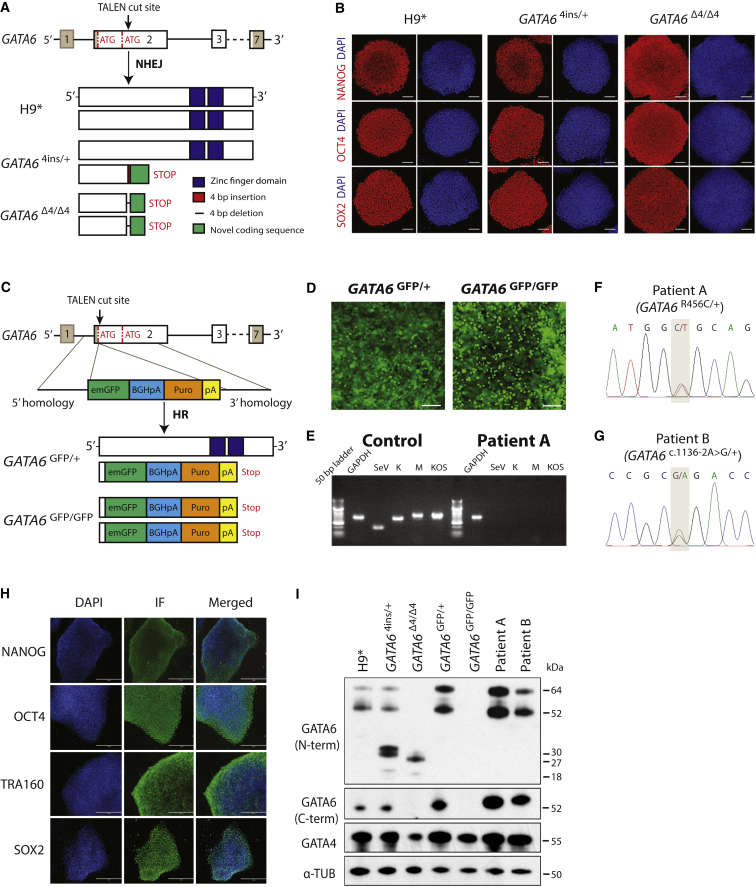
Figure 1.
Derivation and Characterization of GATA6 Mutant Lines
(A) Schematic of the GATA6 locus. Gray shading highlights the 5′ and 3′ untranslated regions. The TALEN cut site lies downstream of the second start ATG in exon 2. Successful gene editing in H9 cells yielded a GATA6 heterozygous line containing a 4-bp insertion (GATA64ins/+) and a homozygous line with an identical 4-bp deletion on each chromosome (GATA6Δ4/Δ4). Each mutation results in the addition of novel coding sequence (green) and a premature stop. H9∗ cells were subjected to gene editing and selection, but have no mutation in GATA6.
(B) OCT4, SOX2, and NANOG immunofluorescence in H9∗, GATA64ins/+, and GATA6Δ4/Δ4 lines confirms pluripotency in gene-edited clones. Scale bars, 100 μm.
(C) A second TALEN cut site downstream of the first ATG in exon 2 of GATA6 is depicted. Cartoon schematic of the “knockin” vector that introduces an emerald GFP (emGFP) reporter in-frame and a puromycin-resistance cassette. Successful homologous recombination resulted in both heterozygous (GATA6GFP/+) and homozygous (GATA6GFP/GFP) mutant cells
(D) Immunofluorescence showing emGFP-expressing heterozygous GATA6GFP/+ and homozygous GATA6GFP/GFP mutant cells on day 3 of differentiation. Scale bars, 100 μm.
(E) PCR showing loss of transgenes in a patient A mutant hiPSC line, clone 1, compared with positive controls. Data are representative of three independent clones derived from either patient A or patient B.
(F and G) Genotype confirmation by Sanger sequencing of two GATA6 patient-derived hiPSC lines: (F) patient A, GATA6R465C/+, and (G) patient B, GATA6c.1136–2A>G/+.
(H) Immunofluorescence confirming the successful reprogramming and pluripotency of one patient A-derived (GATA6R465C/+) mutant line. Scale bars, 200 μm. Images are representative of three independent clones derived from either patient A or patient B (GATA6c.1136–2A>G/+).
(I) Western blot analysis of GATA6 and GATA4 protein levels in undifferentiated H9∗, GATA64ins/+, GATA6Δ4/Δ4, GATA6GFP/+, and GATA6GFP/GFP mutant cells, as well as the two patient-derived mutant lines: patient A, GATA6R465C/+, and patient B, GATA6c.1136–2A>G/+. α-tubulin was used as a loading control. Long and short isoforms of wild-type GATA6 are 60 and 45 kDa, respectively; the partial protein products for GATA64ins/+ are 30 and 18 kDa for the long and short isoforms, respectively; the partial protein products for GATA6Δ4/Δ4 are 27 and 15 kDa for the long and short isoforms, respectively. No GATA6 protein was present for the GATA6GFP/GFP mutant.