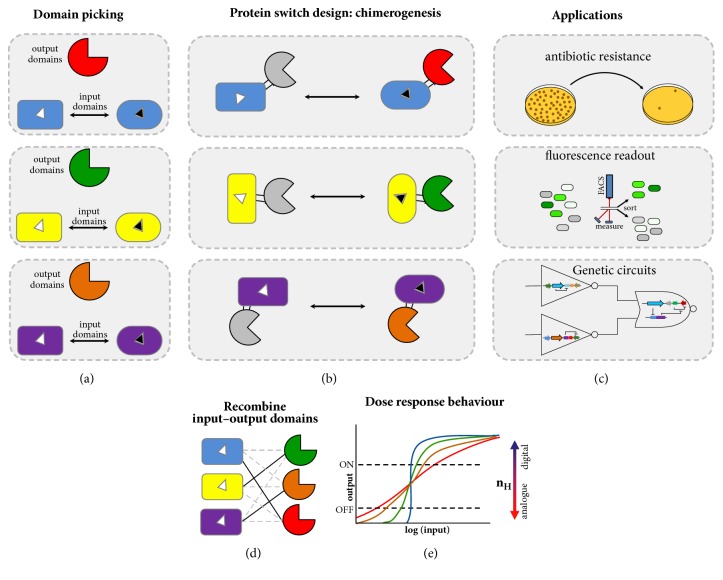
Figure 1.
Schematic depiction of the creation of protein switches by domain insertion. (a) The input or output domains are selected according to the desired application and also structural characteristics. Generally, the protein to be inserted has proximal N- and C-termini. Ligand-mediated conformational changes in the input domain may allow molecular communication between fused domains through conformational coupling. Different colors represent different domains. (b) After chimerogenesis, by domain insertion, the protein switch has the two domains fused in such a way that the activity of the output domain is regulated by the input domain's recognition of an input signal. (c) Depending on the coupled protein functions, the switches can be used as powerful tools for several applications, such as diagnostics, high throughput screenings, and integrating genetic circuits. The grey color of the output domain indicates that the protein is inactive. The signal that modulates the switch is showed as a black triangle. (d) A significant question in the design of novel protein switches is finding the correct combination between the input/output domains which allows the signal/response coupling. (e) According to the molecular switch sensitivity, the protein can show a digital- or analogue-like behavior. Hill coefficients (nH) > 1 show a cooperative response.