Abstract.
Alphaviruses and flaviviruses are known to be endemic in Eastern Africa, but few data are available to evaluate the prevalence of these infections. This leads to missed opportunities for prevention against future outbreaks. This cohort study investigated the frequency of alphavirus and flavivirus incident infections in two regions of Kenya and identified potential risk factors. Seroconversions for alphavirus and flavivirus infections were identified by immunoglobulin G enzyme-linked immunosorbent assay (IgG-ELISA) in a cohort of 1,604 acutely ill children over the year 2015. The annual incidence was 0.5% (0.2–1.2%) for alphaviruses and 1.2% (0.7–2.2%) for flaviviruses. Overall, seroprevalence was significantly higher for alphaviruses in western Kenya than on the coast (P = 0.014), whereas flavivirus seroprevalence was higher on the coast (P = 0.044). Poverty indicators did not emerge as risk factors, but reliance on household water storage was associated with increased exposure to both alphaviruses and flaviviruses (odds ratio = 2.3).
Sub-Saharan African residents are at high risk for several arboviral infections, but regional surveillance data are scarce, and, therefore, the full burden of exposure and disease is unknown. Dengue (DENV, Flavivirus) and chikungunya (CHIKV, Alphavirus) infections are both characterized by an acute febrile syndrome, which can be difficult to distinguish clinically from malaria without effective diagnostics. In an attempt to fill in the gap in knowledge about arboviral transmission, we started a surveillance study in January 2014 among children in Kenya. Whereas the comprehensive study was carried out until June 2018, this preliminary assessment reports results from testing of blood samples obtained during 2015. It characterizes the status of transmission of these viruses, and provides information important to public health institutions. We detected cases of acute febrile illness (AFI) caused by alphaviruses and flaviviruses among children and found a difference in prevalence between coastal and western Kenya. We also identified factors associated with risk of infection: practicing outdoor activity and using unreliable water sources.
We enrolled a cohort of 1- to 17-year-old acutely ill subjects presenting at one of four health-care centers (Kisumu and Chulaimbo in western Kenya, and Ukunda and Msambweni in coastal Kenya). In both regions of Kenya, we selected centers located in an urban area (Kisumu and Ukunda) and a rural area (Chulaimbo and Msambweni). Subjects’ blood was drawn at the initial visit and again during a follow-up visit (3–6 weeks later). Sera from these paired samples were tested by ELISA for CHIKV and DENV IgG. Seroconversion was defined as having negative serum IgG at the initial visit, but a positive serum IgG at the follow-up visit. Although we tested the samples using inactivated DENV and CHIKV as ELISA antigens, antibodies to other flaviviruses and alphaviruses have been reported to cross-react with DENV and CHIKV, respectively.1,2 Because of uncertainty in viral specificity of the antibodies, henceforth, we refer to results from our DENV and CHIKV assays as flavivirus and alphavirus results, respectively. The positive controls used for the ELISAs were made with pooled sera that had neutralizing antibodies for DENV or CHIKV exclusively, without other traces of flavivirus or alphavirus infection (plaque reduction neutralization test). Written informed consent was obtained from the parents of all study subjects and assent from all children older than the age of 7 years. Protocols were approved by the Stanford University Institutional Review Board (IRB-31488) and the Kenya Medical Research Institute Scientific and Ethical Review Unit (protocol 2611). In addition, we collected behavioral and environmental data at each patient visit and performed association tests to identify potential risk factors for alphavirus or flavivirus infections. Seroprevalence and seroincidence 95% confidence intervals (CIs) were calculated using the Wilson/Brown method. Categorical comparisons were tested by chi-square or Fisher’s exact test; continuous comparisons were tested by t-test; and odds ratios (OR) and their CIs were calculated with an online calculator.3
Between December 2014 and December 2015, 1,604 child participants were enrolled in the study, 968 (60%) of whom completed a follow-up visit. Seroprevalence was calculated for the whole cohort, including both acute and follow-up visit results (seropositive at any time point of the study), whereas seroincidence was calculated among those with paired serum samples. More participants were enrolled at the coastal sites (n = 1,118) than at the western sites (n = 486). Western subjects were younger than their coastal counterparts (mean age 4.4 versus 5.9 years, respectively, P < 0.001), with no significant clinical impact on the study however. Fifty-one percentage of the cohort were female, with no difference in the proportion of male and female subjects enrolled at each site (P = 0.77). We identified 12 flavivirus seroconversions (Chulaimbo: 7, Msambweni: 4, and Ukunda: 1) and five alphavirus seroconversions (Kisumu: 1, Msambweni: 1, Ukunda: 3). Flavivirus seroincidence was 1.2% (95% CI: 0.7–2.2%) and alphavirus seroincidence was 0.5% (95% CI: 0.2–1.2%). We observed no significant difference between alphavirus and flavivirus incidence in our cohort (5/968 versus 12/968, respectively, P = 0.14). Ten of the 17 total seroconversions (59%) occurred between January 2015 and February 2015, suggesting an enhanced viral transmission during this period. No concurrent flavivirus/alphavirus coinfection was observed.
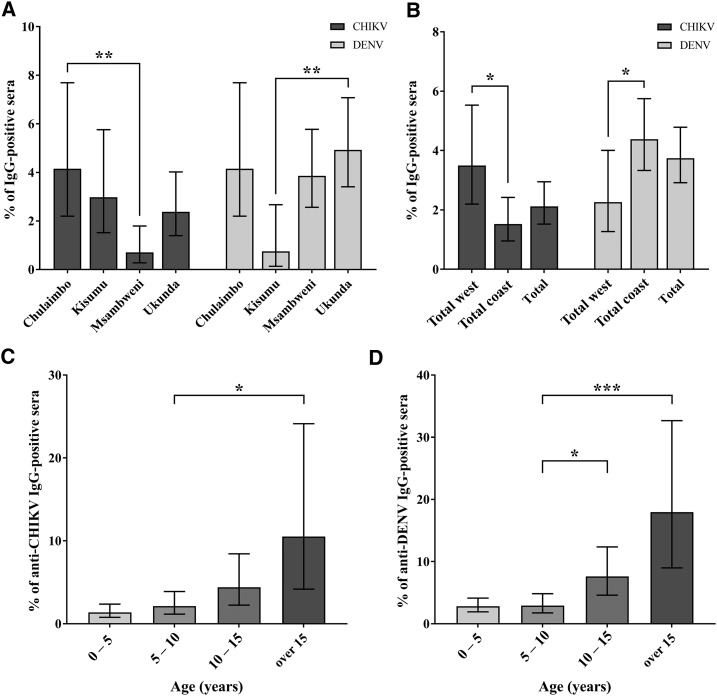
Fisher’s exact test was used to perform the following pairwise comparisons, with an original P-value threshold of α = 0.05, adjusted to α = 0.01 after the Bonferroni correction. Overall, flavivirus seroprevalence (3.7%, 60/1,604) was significantly higher than alphavirus seroprevalence (2.1%, 34/1,604, P = 0.009). Alphavirus seropositivity was more frequent among subjects from western Kenya (3.5%, 17/486) than among subjects from coastal Kenya (1.5%, 17/1,118, P = 0.014, Figure 1B), whereas flavivirus seropositivity was significantly higher among the coastal subjects (4.4%, 49/1,118) than among the western subjects (2.3%, 11/486, P = 0.044, Figure 1B). We observed no difference in flavivirus exposure, comparing urban with rural sites (3.6%, 29/817 versus 3.9%, 31/787, respectively, P = 0.70), or in alphavirus exposure (2.6%, 21/817 versus 1.7%, 13/787, respectively, P = 0.23). As expected, we found that seroprevalence of both viruses increased with age (Figure 1C and D). Moreover, the older group of children we included in this study was composed of secondary school students, and this age proxy was highly associated with a higher seroprevalence for alphaviruses, compared with younger groups (OR = 19.2, 95% CI: 5.7–65.1).
Figure 1.
Alphavirus and flavivirus seroprevalence. Seroprevalence was assessed by anti-IgG ELISA. * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001, all P-values were calculated using pair-wise Fisher’s exact test. (A) Alphavirus, as represented by “chikungunya (CHIKV),” and flavivirus, as represented by “Dengue (DENV),” seroprevalence by study site. (B) Alphavirus and flavivirus seroprevalence by western or coastal location. (C and D) Alphavirus and flavivirus seroprevalence by respective age range.
All household environment and clinical parameters were tested independently for association with alphavirus and flavivirus seropositivity (Table 1). Patients with positive serology at any time point of the study (initial or follow-up visit) were included in the seropositive group. Poverty indicators (not owning a radio, a TV, a motorized vehicle, or a telephone, or not employing a domestic worker) were not significantly associated with alphavirus or flavivirus seroprevalence. Of note, use of water sources requiring intermediate water storage near the household (i.e., inside well; river or pond; rain water; and water truck) instead of public water sources (public tap or well) was significantly associated with higher probability of being exposed to both alphaviruses and flaviviruses (OR = 2.3, 95% CI: 1.0–5.4 and OR = 2.3, 95% CI: 1.2–4.3, respectively). Children who reported outdoor activity were more likely to be infected by both alphaviruses and flavivirus (OR = 3.6, 95% CI: 1.1–11.8 and OR = 2.0, 95% CI: 1.0–3.8, respectively). Also, contrary to common perceptions, children who always slept under a bed net were not significantly protected against alphaviruses (OR = 0.6, 95% CI: 0.3–1.5) or flaviviruses (OR = 1.0, 95% CI: 0.5–1.9) compared with those who never used bed nets or used them only occasionally.
Table 1.
Household environment and exposure to alphavirus and flavivirus infections
| Parameter | Risk factors tested | CHIKV association | DENV association |
|---|---|---|---|
| Behavior and exposure | |||
| Child occupation (proxy for age) | 0–2 years vs. nursery school vs. primary school student vs. secondary school student | P < 0.001 | P < 0.001 |
| Secondary school student vs. other occupations | 19.2 (5.7–65.1) | 1.7 (0.2–12.9) | |
| Outdoor activity | Child reports outdoor activities vs. child does not report outdoor activity | 3.6 (1.1–11.8) | 2.0 (1.0–3.8) |
| Household and supplies | |||
| Number of other children living in the house | 0 vs. 1–2 vs. 3–5 vs. 6 or more | P = 0.46 | P = 0.039 |
| 6 or more vs. 0–5 | – | 1.9 (0.9–3.7) | |
| Roof type | Natural material vs. corrugated iron | 2.8 (1.2–6.1) | 0.7 (0.4–1.1) |
| Floor type | Dirt vs. wood vs. cement vs. tile | P = 0.022 | P = 0.25 |
| Cement vs. other floor types | 3.0 (1.5–6.2) | – | |
| Water source | River or pond vs. rain water vs. public well vs. inside well vs. public tap vs. water truck | P < 0.001 | P = 0.016 |
| (River or pond or rain water or inside well or water truck) vs. (public well or public tap)* | 2.3 (1.0–5.4) | 2.3 (1.2–4.3) | |
| Light source | Electricity line vs. paraffin vs. solar | P = 0.042 | P = 0.37 |
| Electricity line vs. other light sources | 2.6 (1.3–5.2) | – | |
CHIKV = chikungunya virus; DENV = dengue virus; OR = odds ratio. Alphavirus and flavivirus associations are based, respectively, on CHIKV and DENV serological data. Differences between all groups were assessed by n-way Fisher’s exact test (italicized P-values). When a significant difference was noted between all the groups (P-value in bold), OR (in straight letters) was calculated to assess association between the highest risk entry (in bold letters) and alphavirus or flavivirus infection.
* OR was used to compare water sources associated with water storage (in bold) in the household vs. other water sources. P-values and OR in bold are meeting significance (P ≤ 0.05, OR 95% confidence interval ≥ 1).
Alphaviruses and flaviviruses were the probable cause of AFI in about 2% of children tested in this yearlong study. Although these incidence rates are low and cannot be extrapolated to central Kenyan highlands, 2% of AFI in the lowlands of Kenya potentially translates to thousands of missed cases of alphavirus and flavivirus infection per year.4 These are likely underestimates of the true seroincidence, as our study only included acutely ill patients presenting to clinics, excluding mild or asymptomatic cases.5,6 Moreover, investigating seroconversions allowed us to identify only primary infections, as previously infected patients were already IgG positive; thus, this technique does not screen the secondary infections, even with another flavivirus or alphavirus. Interestingly, we documented a significantly higher alphavirus seroprevalence inland than on the Indian Ocean coast, whereas flavivirus seroprevalence exhibited the opposite pattern. These differences have been described in previous studies and suggest that transmission is not only determined by vector abundance but also differs regionally in Kenya.7–10 A better clinical characterization of these infections is desirable to improve the quality of care given to patients.11 More importantly, we know that underlying endemic transmission constitutes a risk for future arboviral epidemics in other areas of Kenya, as it has occurred recently for CHIKV along the coastal islands and for DENV in northern cities and in Mombasa.12–14 Seven (41%) of the 17 seroconversions that we identified occurred during dry periods (monthly rainfall ≤ 50 mm, corresponding to 25% of the months with data collected), which suggests that relative drought increases the risk of exposure to arboviruses in Kenya, as previously shown for CHIKV.15 The use of unreliable water sources and unprotected water storage during these periods seem to have a strong impact on arboviral exposure.16,17
Acknowledgments:
We would like to thank all the participants in our study, the field workers, and the clinicians and staff from the health-care centers in Kenya. We also thank Terence Dermody for giving us the plasmid pSinRep5-181/25ic (Addgene plasmid #60078).
REFERENCES
- 1.Vu DM, et al. 2017. Dengue and West Nile virus transmission in children and adults in coastal Kenya. Am J Trop Med Hyg 96: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBeaud AD, et al. 2015. High rates of O’nyong nyong and chikungunya virus transmission in coastal Kenya. PLoS Negl Trop Dis 9: e0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Select Statistical Services, 2017. Odds Ratio—Confidence Interval—Select Statistical Consultants, 2017. Available at: https://select-statistics.co.uk/calculators/confidence-interval-calculator-odds-ratio. Accessed June 17, 2017.
- 4.O’Meara WP, et al. 2015. Etiology of pediatric fever in western Kenya: a case-control study of falciparum malaria, respiratory viruses, and streptococcal pharyngitis. Am J Trop Med Hyg 92: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafique I, Saqib MAN, Munir MA, Qureshi H, Taseer I-H, Iqbal R, Ahmed W, Akhtar T, Rizwanullah, 2017. Asymptomatic dengue infection in adults of major cities of Pakistan. Asian Pac J Trop Med 10: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 6.Yoon I-K, et al. 2015. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis 9: e0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland LJ, Cash AA, Huang Y-JS, Sang RC, Malhotra I, Moormann AM, King CL, Weaver SC, King CH, LaBeaud AD, 2011. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg 85: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mease LE, Coldren RL, Musila LA, Prosser T, Ogolla F, Ofula VO, Schoepp RJ, Rossi CA, Adungo N, 2011. Seroprevalence and distribution of arboviral infections among rural Kenyan adults: a cross-sectional study. Virol J 8: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cromwell EA, Stoddard ST, Barker CM, Van Rie A, Messer WB, Meshnick SR, Morrison AC, Scott TW, 2017. The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infection. PLoS Negl Trop Dis 11: e0005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowman LR, Runge-Ranzinger S, McCall PJ, 2014. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis 8: e2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu DM, Mutai N, Heath CJ, Vulule JM, Mutuku FM, Ndenga BA, LaBeaud AD, 2017. Unrecognized dengue virus infections in children, 2014–2015. Emerg Infect Dis 23: 1915–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sang RC, Dunster LM, 2001. The growing threat of arbovirus transmission and outbreaks in Kenya: a review. East Afr Med J 78: 655–661. [DOI] [PubMed] [Google Scholar]
- 13.Kariuki Njenga M, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CHL, Sang R, Sergon K, Breiman R, Powers AM, 2008. Tracking epidemic chikungunya virus into the Indian ocean from east Africa. J Gen Virol 89: 2754–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutomiah J, et al. 2016. Dengue outbreak in Mombasa city, Kenya, 2013–2014: entomologic investigations. PLoS Negl Trop Dis 10: e0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chretien J-P, et al. 2007. Drought-associated chikungunya emergence along coastal east Africa. Am J Trop Med Hyg 76: 405–407. [PubMed] [Google Scholar]
- 16.Ngugi HN, et al. 2017. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors 10: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndenga BA, Mutuku FM, Ngugi HN, Mbakaya JO, Aswani P, Musunzaji PS, Vulule J, Mukoko D, Kitron U, LaBeaud AD, 2017. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS One 12: e0189971. [DOI] [PMC free article] [PubMed] [Google Scholar]