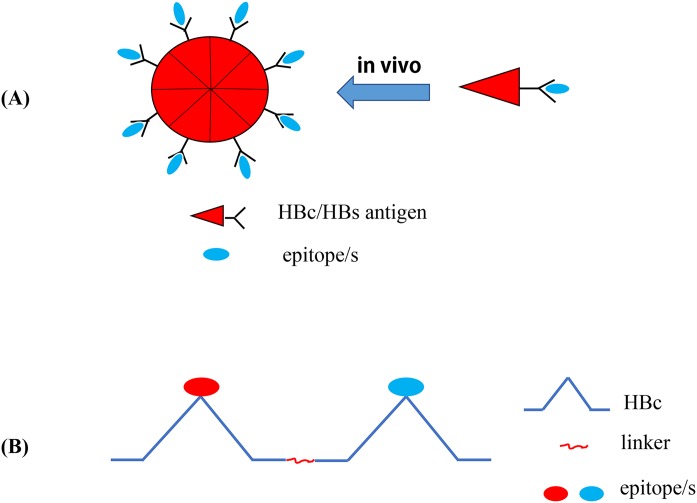
Figure 4. Recombinant HBc-based VLPs or HBs-based VLPs.
(A) (1) The HBc proteins naturally form the dimers, the building blocks that forms the VLPs. It takes about 60 such dimers (i.e., 120 copies of HBc) to form a HBc-based VLP. The results showed that there were about 40 amino acid residues inserted into the N-terminal of HBc. In the MIR region of HBc, 50 or 100 amino acid residues can be inserted, and as many as 100 or more residues at the C-terminal do not interfere with the formation of particles. (2) Hepatitis B surface antigen (HBsAg) can also self-assemble into highly organized viroid particles with a diameter of 22 nm. These HBs-derived VLPs contain about 100 HBsAg molecules and provide a unique opportunity to display multiple exogenous epitopes. (B) Hepatitis B virus tandem core platform. The two replicas of HBc protein are linked together by covalent bonds through flexible amino acid sequences so that the fused dimers can be folded correctly and assembled into HBc particles. In the assembled HBc particles, the four helix bundles formed at each dimer interface appear on the surface as prominent “spikes”. The tip of the spike is the preferred site for inserting foreign sequences for bivalent vaccine.