Abstract
A total of 16 different strains of Microbacterium spp. were isolated from contaminated soil and enriched on the carcinogen, hexavalent chromium [Cr(VI)]. The majority of the isolates (11 of the 16) were able to tolerate concentrations (0.1 mM) of cobalt, cadmium, and nickel, in addition to Cr(VI) (0.5–20 mM). Interestingly, these bacteria were also able to tolerate three different antibiotics (ranges: ampicillin 0–16 μg ml−1, chloramphenicol 0–24 μg ml−1, and vancomycin 0–24 μg ml−1). To gain genetic insight into these tolerance pathways, the genomes of these isolates were assembled and annotated. The genomes of these isolates not only have some shared genes (core genome) but also have a large amount of variability. The genomes also contained an annotated Cr(VI) reductase (chrR) that could be related to Cr(VI) reduction. Further, various heavy metal tolerance (e.g., Co/Zn/Cd efflux system) and antibiotic resistance genes were identified, which provide insight into the isolates’ ability to tolerate metals and antibiotics. Overall, these isolates showed a wide range of tolerances to heavy metals and antibiotics and genetic diversity, which was likely required of this population to thrive in a contaminated environment.
Keywords: Microbacterium, Chromium reduction, Genomics, Antibiotic resistance, Heavy metals
Introduction
Heavy metals, while naturally occurring, cause harm to both human and ecosystem health. Specifically, chromium is a mutagen and carcinogen and its presence in the environment can be natural due to anthropogenic activities, such as industrial manufacturing (e.g., metal plating and tanneries) and mining (chromite ore) (Barak et al., 2006; Brose & James, 2010; Cheng, Holman & Lin, 2012). In the environment, chromium can exist as hexavalent chromium [Cr(VI)], which is soluble and more toxic, or as insoluble trivalent chromium [Cr(III)] (Bartlett, 1991; Cheng, Holman & Lin, 2012). Thus, the redox cycling of chromium is critical to understanding its impacts on the environment. In natural systems, Cr(III) can be oxidized by manganese oxides and hydrogen peroxide, while Cr(VI) can be reduced by ferrous iron and hydrogen sulfides (Brose & James, 2010; Oze et al., 2004; Viti et al., 2013).
Many bacteria are able to tolerate Cr(VI) stress based on their ability to transport it outside the cell or enzymatically reduce it to the less toxic form Cr(III). The efflux pump, chrA, has been associated with providing Cr(VI) resistance by transporting it outside the cell (Cervantes & Ohtake, 1988; Cervantes et al., 1990; Nies, Nies & Silver, 1990). A 2008 study found 135 chrA orthologs of this efflux pump (Ramirez-Diaz et al., 2008); however, the presence of chrA is not always a sole predictor of the amount of Cr(VI) that bacteria can resist (Henne et al., 2009a). Bacteria can also have additional efflux pumps that provide resistance to other metals (Nies, 2003; Silver, 1996), which is advantageous as anthropogenically impacted sites are often contaminated with multiple stressors. Further, there is a well-known association between metal and antibiotic tolerance (Baker-Austin et al., 2006; Seiler & Berendonk, 2012; Wright et al., 2006). For example, Staphylococcus species use multiple efflux pumps to tolerate chromium, lead, and penicillin (Ghosh et al., 2000) and Salmonella abortus equi strains were able to tolerate chromium, cadmium, mercury, and ampicillin (Ug & Ceylan, 2003). Thus, efflux pumps can play a vital role in how bacteria thrive in contaminated ecosystems.
Bacteria are also known to catalyze the reduction of Cr(VI) aerobically. Specifically, certain bacteria use a soluble NADH/NADPH-dependent oxidoreductase to reduce Cr(VI) (Ackerley et al., 2004a; Barak et al., 2006; Cheung & Gu, 2007; Gonzalez et al., 2005; Park et al., 2000). Two well-studied Cr(VI) reductases are chrR and yieF. In Pseudomonas putida, chrR reduces Cr(VI) via the transfer of one electron, generating Cr(V), a reactive intermediate, which then requires a second electron transfer to generate the more stable insoluble Cr(III) (Park et al., 2000). Conversely, Escherichia coli uses yieF to catalyze the transfer of four electrons to reduce Cr(VI) (Ackerley et al., 2004b; Barak et al., 2006; Ramirez-Diaz et al., 2008).
The objective of this study was to examine the genomes of 16 Microbacterium spp. strains isolated from metal contaminated soil to identify their genetic potential and physiological ability to reduce Cr(VI) and to tolerate both heavy metals and antibiotics. A recent genomic study of four Cr(VI) reducing Microbacterium spp. identified two putative reductases (Henson et al., 2015), one genetically similar to chrR in Thermus scotoductus (Opperman, Piater & van Heerden, 2008) and the other to yieF of Arthrobacter sp. RUE61a (Niewerth et al., 2012); however, these putative reductases had low sequence similarity to the well-studied reductases found in Pseudomonas putida and E. coli. While other studies have shown Microbacterium isolates are able to reduce Cr(VI) (Humphries et al., 2005; Liu et al., 2012; Pattanapipitpaisal, Brown & Macaskie, 2001; Soni et al., 2014), there are still unknowns related to the genetics of Cr(VI) reduction and resistance. Further, the study by Henson et al. (2015) documented high levels of genomic variability between the isolates, which might be related to ecotypes. However, the conclusions of this study were limited based on the low number of genomes examined. Comparative genomics has been used in various environments and microorganisms to examine inter-strain variation and ecotypes (Briand et al., 2009; Coleman & Chisholm, 2010; Denef et al., 2010; Frangeul et al., 2008; Humbert et al., 2013; Martiny, Coleman & Chisholm, 2006; Meyer & Huber, 2014; Meyer et al., 2017). Thus, this study examined the genomic and physiological variability of heavy metal and antibiotic resistance of 16 Microbacterium strains from the same contaminated soil environment to elucidate the potential genomic flexibility of closely related strains.
Methods
Bacterial isolation
Isolation of Microbacterium spp. from soil is described in Kourtev, Nakatsu & Konopka (2009) and Henson et al. (Henson et al., 2015). Bill Jervis from the Indiana Department of Transport provided site access (no permit was required) and the project did not involve endangered or protected species. Briefly, contaminated soil samples were collected from the Department of Transportation site in Seymour, IN, USA. Previous studies have documented the site was contaminated with Pb (1,156 μg−1 soil), Cr (5,868 μg−1 soil), and hydrocarbons (toluene and xylenes: >200 μg g−1 soil) (Joynt et al., 2006; Kourtev, Nakatsu & Konopka, 2006; Nakatsu et al., 2005). Bacteria were initially isolated from the soil on 50% tryptic soy agar amended with 0.25 mM Cr(VI) (K2CrO4). From this, 16 isolates were further selected based on their ability to grow on at least 0.5 mM Cr(VI). The isolates were stored in glycerol stocks at −80 °C until further use.
Cr(VI) reduction
For Cr(VI) reduction experiments, isolates were grown in 25% tryptic soy broth (TSB) with 0.5 or 1 mM Cr(VI) at 30°C and 225 rpm for 72 h. Two concentrations were used as certain isolates had relatively lower growth at 1 mM compared to other isolates. The cultures were pelleted and Cr(VI) reduction was determined using a colorimetric assay (Henson et al., 2015; Urone, 1955).
DNA sequencing and bioinformatics
Isolates were grown in TSB with 0.5 or 2 mM Cr(VI) at 30 °C and 225 rpm for 24–72 h. The cultures were pelleted via centrifugation and stored at −20 °C until used in DNA extractions. FastDNA Spin Kits (MP Biomedical) were used to extract genomic DNA from the thawed pellets. The resulting DNA was stored at −20 °C. The DNA from the 16 bacterial isolates were sent to Cincinnati Children’s Hospital Medical Center’s Genetic Variation and Gene Discovery Core facility for whole genome shotgun sequencing using one lane of an Illumina HiSeq 2000 (100bp PE). The resulting raw genomic reads can be found in the National Center for Biotechnology Information’s (NCBI) Short Read Archives (SRA), accession number: SRP120551.
Raw reads were evaluated for quality with FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and trimmed and quality filtered with Trimmomatic (v 0.33) (Bolger, Lohse & Usadel, 2014). Sequence quality scores were low on R2 (assessed by FastQC), so the trimmed reads were cut (length of 70bp) and passed through another quality filter (-Q33 -q 30 -p 50) with FastX toolkit (http://hannonlab.cshl.edu/fastx_toolkit/). Reads for each sample were subsampled to four million reads (multiple subsampling depths were tested) with seqtk (https://github.com/lh3/seqtk). Using multiple different read depths, MEGAhit (Li et al., 2016) was used to assemble the data, which were then assessed for quality with QUAST (Gurevich et al., 2013). CheckM (Parks et al., 2015) was used to examine genome completeness and contamination (taxon marker Microbacterium). The resultant genomes were annotated by the Department of Energy’s Joint Genome Institute Integrated Microbial Genomes (IMG) system (Markowitz et al., 2012) and are publicly available (see Table S1). A pangenomic analysis of 20 genomes (from this study and Henson et al., 2015) was conducted using the An’vio program (version 3) (Eren et al., 2015) following the pangenomics workflow from Delmont & Eren (2018). The pangenomic analysis within An’vio also utilized other programs like HMMER (Eddy, 2011), Prodigal (Hyatt et al., 2010), and NCBI’s blastp (Altschul et al., 1997). An’vio was also used to define clusters of orthologous groups (COGs) for the pangenomic analysis using the command anvi-run-ncbi-cogs.
All 16, 16S rRNA gene sequences from the Cr(VI) isolates were downloaded from IMG. These genes were used to generate a maximum likelihood phylogenetic tree in MEGA7 (Kumar, Stecher & Tamura, 2016) and evolutionary history was inferred using the maximum likelihood Tamura–Nei model (Tamura & Nei, 1993). Within IMG, annotations were searched for genes related to Cr(VI) resistance and Cr(VI) reduction via keywords (e.g., efflux, reductase, and chromate). Annotated Cr(VI) reductase genes were compared via BLASTP (one-way comparison on NCBI’s website) to a known Cr(VI) reducing chrR from Pseudomonas putida KT2440 (NP_746257) (Ackerley et al., 2004b). Genes related to heavy metal resistance and antibiotic resistance were searched using functional categories. Beta-lactam, chloramphenicol, and vancomycin resistance genes were also identified using KEGG pathway KO identifiers. The COG category “Inorganic ion transport and metabolism” was used to find annotated heavy metal efflux genes and metal resistance genes.
Metal and antibiotic tolerance assays
Bacterial isolates were grown on agar plates (10% TSB, 1.5% agar, and 0.5 mM Cr(VI)) at 30 °C until colonies were visible. Colonies were then streaked onto fresh agar plates amended with different concentrations of heavy metals (Cd 0–5 mM, Co 0–5 mM, Cr 0–20 mM, Cu 0–1 mM, Ni 0–15 mM, and Zn 0–1 mM). Duplicate plates were incubated at 30 °C for 14 days and growth was confirmed by the presence of visible colonies. All bacterial isolates were plated on each metal concentration in duplicate.
The Minimum Inhibitory Concentration (MIC) for ampicillin, chloramphenicol, and vancomycin was tested on each isolate using MIC test strips (Liofilchem®). To do this, each of the 16 Microbacterium spp. isolates was streaked onto agar plates (50% TSB and 1.5% agar) with 0.5 mM Cr and incubated at 30 °C for 5 days. Next, each isolate was suspended in a 0.85% sterile saline solution until it reached a density that approximated the 0.5 McFarland Turbidity Standard. The isolates were then spread uniformly on a Mueller–Hinton agar plate. A MIC test strip was placed in the center of each plate. The plates were incubated at 30 °C for 24 h. After the 24 h, the plates were removed and the MIC of each isolate to each antibiotic was determined by visually documenting the zones of inhibition.
Results and Discussion
Isolation of Cr(VI) reducing bacteria
Though originally isolated and studied for their ability to reduce Cr(VI) (Henson et al., 2015; Kourtev, Nakatsu & Konopka, 2009), it was speculated that these isolates may also be tolerant to other contaminants, because the soils were contaminated with other heavy metals and organic solvents (Joynt et al., 2006; Kourtev, Nakatsu & Konopka, 2006; Nakatsu et al., 2005). Among the 16 isolates studied here, a wide range of Cr(VI) tolerance and reducing ability was found (Table 1). The majority (13) of the isolates could tolerate two mM Cr(VI), while three of the isolates (K2B2, K36, and K40) could only tolerate 0.5 mM Cr(VI). Interestingly, five of the 13 isolates that were able to tolerate two mM Cr(VI) (A20, K24, K30, K33, and PF3) had observable colonies on plates containing up to 20 mM Cr(VI). Further, the 16 isolates also showed a wide range in Cr(VI) reduction (0–88.8%) over a 72-h incubation (Table 1). Only two isolates, K27 and K33, reduced over 80% of the Cr(VI) in the experiment. Interestingly, K27 reduced 83% of the Cr(VI) in liquid medium but was only able to tolerate 0.5 mM Cr(VI) when grown on solid medium, while K33 tolerated up to 20 mM Cr(IV) and reduced 88% of the Cr(IV) present. Two additional isolates, K31 and PF3, reduced over 50% of the Cr(VI) in the experiment. There was no statistical relationship between the ability of isolates to tolerate and their ability to reduce Cr(VI).
Table 1. Heavy metal tolerance and chromate reduction.
| Metal tolerance (mM) | |||||||
|---|---|---|---|---|---|---|---|
| Isolate | Cr | Ni | Co | Cd | Zn | Cu | Cr reduction (%)* |
| Microbacterium sp. A20 | 20.0 | 1.0 | 0.1 | 0.1 | 1.0 | 1.0 | 41.6 |
| Microbacterium sp. K19 | 2.0 | 1.0 | 0.1 | 0.1 | 1.0 | 1.0 | 34.0 |
| Microbacterium sp. K21 | 2.0 | 1.0 | 0.1 | 0.1 | 1.0 | 1.0 | 39.3 |
| Microbacterium sp. K22 | 2.0 | 1.0 | 0.1 | 0.1 | 0.1 | 1.0 | 42.9 |
| Microbacterium sp. K24 | 20.0 | 1.0 | 1.0 | 0.0 | 1.0 | 1.0 | 41.6 |
| Microbacterium sp. K27 | 2.0 | 1.0 | 1.0 | 0.1 | 0.1 | 1.0 | 83.0 |
| Microbacterium sp. K2B2 | 0.5 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 36.0 |
| Microbacterium sp. K30 | 20.0 | 1.0 | 1.0 | 0.1 | 1.0 | 1.0 | 41.6 |
| Microbacterium sp. K31 | 2.0 | 0.1 | 0.1 | 0.1 | 1.0 | 1.0 | 55.3 |
| Microbacterium sp. K33 | 20.0 | 1.0 | 0.1 | 0.1 | 1.0 | 1.0 | 88.8 |
| Microbacterium sp. K35 | 2.0 | 0.1 | 0.1 | 0.1 | 0.1 | 1.0 | 3.6 |
| Microbacterium sp. K36 | 0.5 | 1.0 | 0.1 | 0.1 | 0.1 | 1.0 | 10.7 |
| Microbacterium sp. K40 | 0.5 | 1.0 | 0.1 | 0.1 | 1.0 | 1.0 | 30.3 |
| Microbacterium sp. K41 | 2.0 | 1.0 | 0.1 | 0.0 | 0.1 | 1.0 | 28.1 |
| Microbacterium sp. K5D | 2.0 | 1.0 | 0.1 | 0.0 | 0.1 | 1.0 | 0.0 |
| Microbacterium sp. PF3 | 20.0 | 1.0 | 0.1 | 0.0 | 1.0 | 1.0 | 50.6 |
Notes:
Heavy metal (cadmium, chromium, cobalt, copper, nickel, and zinc) tolerance (in mM) of each isolate and chromate reduction (%) data.
Reduction % after 72 h with one mM Cr except for K19, 31, 27 which was 0.5 mM.
Data shown in percentages since two chromate concentrations were used.
Genomic assembly and pangenomic analysis
Previous research has shown Cr(VI) tolerance to be related to a resistance gene (chrA) (Cervantes & Ohtake, 1988; Cervantes et al., 1990) and/or Cr(VI) reductases (chrR and yieF; (Ackerley et al., 2004b; Barak et al., 2006; Park et al., 2000; Ramirez-Diaz et al., 2008). To gain a better understanding of the Cr(VI) tolerance of these isolates, their genomes were assembled and annotated. The isolate genomes ranged in total length from 3.31 to 4.23 Mb and all had GC content ranging from 68 to 70%% (Table 2). A genomic study of 10 Macrobacterium sp. documented similar GC ranges and genome length (Corretto et al., 2015). The assembled genomes were generally over 96.7% complete and all genomes had under 4% contamination (Table 2).
Table 2. Assembly statistics and quality assurance data.
| Isolate | Total length (Mb) | GC (%) | Number of contigs | Largest contig (bp) | N50 (bp) | Est. Sequencing coverage | Comp. (%) | Cont. (%) | Strain heterogeneity (%) |
|---|---|---|---|---|---|---|---|---|---|
| Microbacterium sp. A20 | 3.94 | 68.53 | 75 | 436,954 | 166,569 | 142 | 99.06 | 0.54 | 0 |
| Microbacterium sp. K19 | 3.89 | 68.69 | 54 | 479,964 | 187,157 | 144 | 99.44 | 1.01 | 20 |
| Microbacterium sp. K21 | 3.85 | 68.33 | 34 | 537,739 | 330,631 | 146 | 99.06 | 0.18 | 0 |
| Microbacterium sp. K22 | 3.94 | 68.52 | 96 | 258,133 | 83,317 | 142 | 99.06 | 0.71 | 33.33 |
| Microbacterium sp. K24 | 4.23 | 68.90 | 142 | 248,507 | 56,118 | 132 | 99.35 | 3.93 | 9.09 |
| Microbacterium sp. K27 | 3.75 | 68.51 | 37 | 342,064 | 187,856 | 149 | 98.66 | 0.18 | 0 |
| Microbacterium sp. K2B2 | 3.94 | 68.52 | 76 | 436,909 | 120,725 | 142 | 99.06 | 0.54 | 0 |
| Microbacterium sp. K30 | 4.06 | 69.16 | 96 | 295,140 | 88,745 | 138 | 98.39 | 2.61 | 0 |
| Microbacterium sp. K31 | 3.77 | 68.43 | 39 | 443,452 | 183,886 | 148 | 98.66 | 0.18 | 0 |
| Microbacterium sp. K33 | 3.89 | 68.68 | 110 | 311,089 | 66,684 | 144 | 99.44 | 1.49 | 0 |
| Microbacterium sp. K35 | 3.62 | 70.91 | 379 | 168,068 | 17,080 | 155 | 96.70 | 1.82 | 12.5 |
| Microbacterium sp. K36 | 3.31 | 70.53 | 91 | 183,861 | 73,738 | 169 | 98.10 | 0.60 | 0 |
| Microbacterium sp. K40 | 3.77 | 68.40 | 45 | 442,332 | 195,355 | 148 | 99.06 | 0.09 | 0 |
| Microbacterium sp. K41 | 3.62 | 70.75 | 454 | 89,969 | 15,114 | 155 | 97.62 | 1.37 | 0 |
| Microbacterium sp. K5D | 3.80 | 68.35 | 49 | 442,284 | 195,368 | 147 | 99.06 | 0.09 | 0 |
| Microbacterium sp. PF5 | 3.74 | 70.80 | 144 | 125,065 | 47,267 | 150 | 97.23 | 1.82 | 0 |
Note:
Genome assembly statistics (total length, GC%, and number of contigs) and quality assurance data (completeness, contamination, and strain heterogeneity).
Phylogenetic analysis of the 16S rRNA gene indicated that all of the isolates belong to the genus Microbacterium (Fig. S1; Table S2). Specifically, the isolates were closely related to Microbacterium oxydans, Microbacterium maritypicum, or Microbacterium paraoxydans. Microbacterium spp. have been isolated from sites contaminated with metals (Avramov et al., 2016; Bollmann et al., 2010; Corretto et al., 2015), radionuclides (Nedelkova et al., 2007), and petroleum (Avramov et al., 2016; Chauhan et al., 2013; Wang et al., 2014). Moreover, some environmental isolates, such as Microbacterium laevaniformans strain OR221, have shown to tolerate multiple metals (Ni, Co, and Cd) (Bollmann et al., 2010). A genomic analysis of the latter strain provided evidence of genes (e.g., transporter and detoxification genes) that could aid the strain’s ability to tolerate metals (Brown et al., 2012). Other Microbacterium spp. have been known to reduce Cr(VI): Microbacterium sp. SUCR140 (Soni et al., 2014), Microbacterium sp. chr-3 (Focardi, Pepi & Focardi, 2013), and Microbacterium sp. CR-07 (Liu et al., 2012).
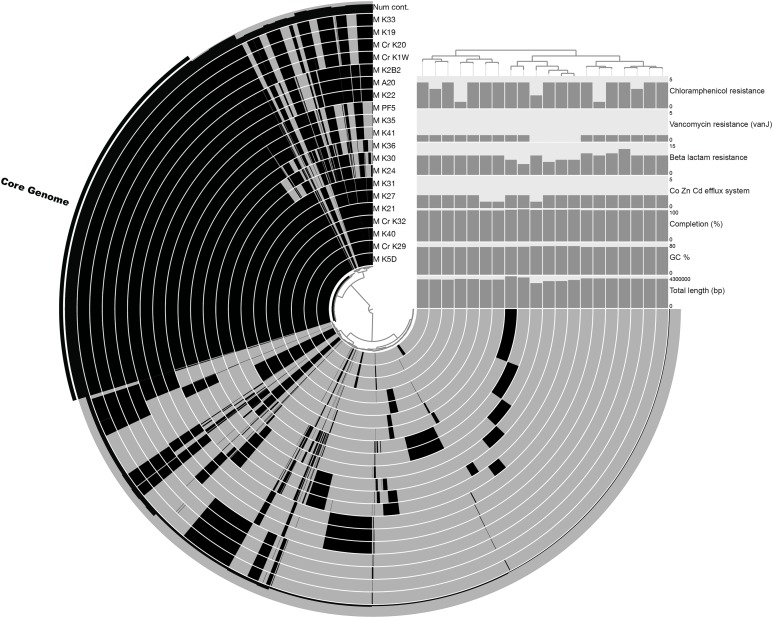
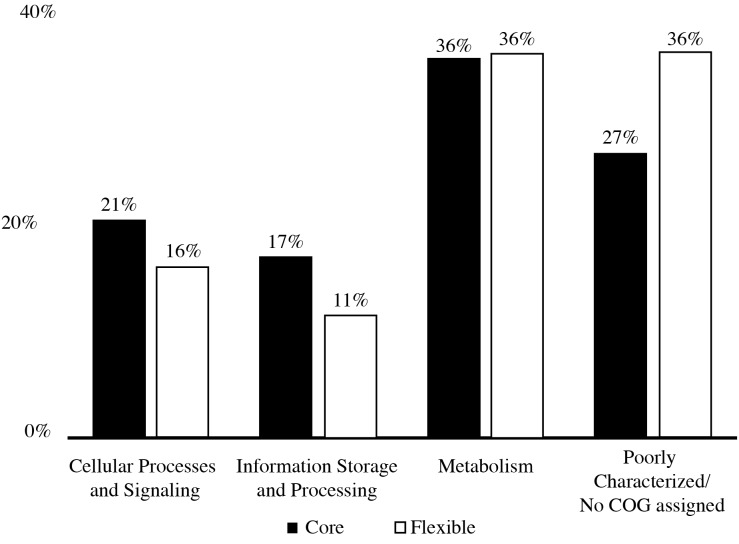
The pan-genome size of the 20 isolates was 7,902 protein clusters with the core genome encompassing 2,073 protein clusters (26%) (Fig. 1). The majority of the genes in the core genome of the 20 isolates (36%) were placed in the “Metabolism” COG (Fig. 2). Genes in the COG categories “Cellular processes and signaling” and “Information storage and processing” comprised 21% and 17%, respectively of the core genome (Fig. 2). The flexible pangenome was dominated by genes in the COG categories “Metabolism” and “Poorly characterized/No assigned COG” (36% for both) (Fig. 2). Relative to the core pangenome, the flexible pangenome had few genes in the COG categories “Cellular processes and signaling (21%)” and “Information storage and processing 17%” (Fig. 2). The percentage of core genes in a genome can vary from 3% to 84% (McInerney, McNally & O’Connell, 2017). For example, the Bacillus cereus core genome is 27% of the pangenome, whereas the core genome of Bacillus anthracis is 65% (McInerney, McNally & O’Connell, 2017). An analysis of 20 Microcystis sp. showed the genome was comprised of 34–49% core genes and 51–66% flexible genes (Meyer et al., 2017). A study on the genomes of 12 Prochlorococcus isolates found a core genome ranging from 40% to 67% (Kettler et al., 2007). The relatively large genomic variability seen with the Microbacterium spp. examined here could be due to selective pressures that drove gene loss or horizontal gene transfer, which in the end enhanced the ability of the isolates to survive in a contaminated sediment environment.
Figure 1. Pangenomic analysis of heavy metal tolerant Microbacterium spp.
The figure was made in An’vio with the items order in presence/absence (D: Euclidean; L: Ward) and the samples ordered by PC frequency. The outer most complete ring (labeled Num cont.) represents the number of genomes that a gene occurs in, with the core genome highlighted with an additional black bar. The internal rings represent an isolate’s genome with the black color representing the presence of genes in a genome (gray is the absence of a gene). The bar charts show quality control measurements (total genome length [base pairs], GC content, and percent completion) and also the abundance of genes that were annotated as Co, Zn, Cd efflux system genes and antibiotic tolerance genes (beta lactam, vancomycin, and chloramphenicol resistance).
Figure 2. COGs of share genes.
COG distribution of genes found in the core and flexible genomes of 20 Microbacterium isolates isolated from heavy metal contaminated soil.
The COG categories “Inorganic ion transport and metabolism (P)” and “Self-defense mechanism (V)” were examined due to their relation to heavy metal and antibiotic transport. The flexible genome has relatively higher percentages of genes in both the self-defense (1% of the core genome and 3% of the flexible genome) and inorganic ion transport and metabolism (5% of the core genome and 6% of the flexible genome) COG category. Each of these COG categories includes predicted gene functions for heavy metal transport and antimicrobial resistance and antibiotic efflux/transporters (Fig. 1). Interestingly, the core and flexible genomes contain different Co/Zn/Cd efflux system component genes and multidrug efflux pump genes, which suggest tolerance to heavy metals and antibiotics. The variability found here may be related to the ability of each isolate to tolerate different types and concentration of heavy metals and antibiotics.
Genomic insights of Cr(VI) reduction
The annotated genomes from this study provided evidence that these isolates could reduce Cr(VI). All possess annotated Cr(VI) reductase, chrR (Table S3). In addition, M. sp K24 and K30 contained two annotated chrR genes. The Cr(VI) reductases were compared with a known Cr(VI) reductase from Pseudomonas putida KT2440 (Park et al., 2000) and BLASTP results showed that all the Microbacterium spp. chrR genes shared a high degree of homology (ranging from 40% to 46% identity) (Table S3). Thus, it is likely that these chrR genes are responsible for the Cr(VI) reduction ability of these isolates. Interestingly, other Microbacterium spp. isolated from this site did not have annotated Cr(VI) reductases but BLAST searches were able to identify genes homologous to chrR and yeiF (Henson et al., 2015), further suggesting high interspecies genetic diversity in the putative Microbacterium spp. Cr(VI) reductases found in the same soil.
Cr(VI) tolerance might not be related to efflux pumps and Cr(VI) reductases. While the genomes did contain Cr(VI) specific reductases, they did not contain a Cr(VI) efflux pump (chrA). The lack of an assembled and annotated chrA was interesting since the ability of the isolates to reduce and resist Cr(VI) did not correlate. While the lack of an assembled chrA does not confirm its absence from the genome, tolerance could be related to other genes. Cr(IV) tolerance has been shown to be related genes that involve oxidative stress response (Ackerley et al., 2004a; Cheng et al., 2009), DNA repair (Hu et al., 2005; Miranda et al., 2005), and metabolism (Brown et al., 2006; Decorosi et al., 2009; Henne et al., 2009b).
Tolerance to multiple heavy metals and antibiotics
The isolates’ genomes all contained numerous genes that suggest these bacteria can tolerate multiple heavy metals (e.g., Co, Zn, and Cd) and antibiotics. When examining KEGG pathways that related to transport, all of the isolates had genes that were annotated to be Co/Zn/Cd efflux system components (Table S4). In addition, some isolates had a putative cadmium resistance protein (Table S4). KEGG pathways also documented genes that could provide tolerance to three different antibiotics (ampicillin, chloramphenicol, and vancomycin) (Table S4).
The isolates’ physiological ability to tolerate heavy metals were examined to determine whether the inferred resistance based on the putative metal transport genes could be confirmed. All the isolates were streaked onto plates with various concentrations of Cd, Co, Cr, Cu, Ni, and Zn. Overall, 11 of the 16 isolates tolerated between 0.1 and 1.0 mM of all six tested metals (Table 1). All 16 isolates were able to tolerate Cr, Ni, Zn, and Cu (Table 1). As the isolates were enriched with Cr, it was expected that they would be able to tolerate higher concentrations of Cr. Overall, the bacteria isolated in this study were able to tolerate many heavy metals, a finding seen in other studies. Microbacterium spp. enriched from Ni-rich serpentine soils have been previously documented to tolerate multiple heavy metals including Cd (0.5–2.5 mM), Co (0.1–5 mM), Cr (1–5 mM), Cu (0.25–10 mM), Ni (5–15 mM), and Zn (5–10 mM) (Abou-Shanab, Van Berkum & Angle, 2007). Another study found that multiple isolates closely related to M. oxydans were able to tolerate Cu (0.25–16 mM), Cr (0.5–16 mM), and Ni (0.25–16 mM) (Nedelkova et al., 2007). As Microbacterium spp. have been isolated from contaminated environments, it is not surprising to find they can tolerate heavy metal contamination. While this study did not attempt to confirm the function of the Co/Zn/Cd efflux system components found in these genomes (Table S4), the presence of these genes could be related to the ability of these isolates to tolerate multiple heavy metals. Whether in culture (e.g., Pseudomonas putida or Cupriavidus necator, formerly Alcaligenes eutrophus) (Manara et al., 2012; Nies, 1995) or in soil microcosms (Cabral et al., 2016), cobalt-zinc-cadmium efflux system proteins have been shown to provide tolerance to heavy metals. Thus, it is possible that the presence of these genes plays the same role in the isolates documented in this study.
There is a long history of research linking metal tolerance and antibiotic resistance (Baker-Austin et al., 2006; Calomiris, Armstrong & Seidler, 1984; Henriques et al., 2016; Seiler & Berendonk, 2012). In this study, 13 of the 16 isolates showed tolerance to ampicillin, chloramphenicol, and vancomycin (Table 3). Specifically, 13 out of 16 isolates were able to grow in the presence of ampicillin with tolerances ranging from 1.5 to 16 μg ml−1 (Table 3). Chloramphenicol tolerance was found in 15 out of 16 isolates, of which four isolates, K24, K35, K36, and K5D, tolerated 24 μg ml−1 of the antibiotic (Table 3). Microbacterium isolates have been previously documented to tolerate various antibiotics. Microbacterium isolates from fish mucus have shown high antibiotic resistance to both ampicillin (>1,600 μg ml−1) and chloramphenicol (> 960 μg ml−1) (Ozaktas, Taskin & Gozen, 2012). Human isolated Microbacterium spp. have been reported to grow in the presence of vancomycin in concentrations ranging from 0.25 to 15 μg ml−1 (Gneiding, Frodl & Funke, 2008). Other clinical Microbacterium isolates had MIC for ampicillin from 1 to 1.5 μg ml−1 and vancomycin from 3 to 4 μg ml−1 (Laffineur et al., 2003). A 2015 study found 26% of Microbacterium isolates were resistant to vancomycin (Bernard & Pacheco, 2015).
Table 3. Antibiotic minimum inhibitory concentrations.
| Isolates | Amp* | Cam* | Van* |
|---|---|---|---|
| Microbacterium sp. A20 | 0.0 | 8.0 | 0.5 |
| Microbacterium sp. K19 | 3.0 | 12.0 | 3.0 |
| Microbacterium sp. K21 | 3.0 | 12.0 | 4.0 |
| Microbacterium sp. K22 | 1.5 | 1.5 | 1.0 |
| Microbacterium sp. K24 | 3.0 | 24.0 | 6.0 |
| Microbacterium sp. K27 | 0.0 | 12.0 | 4.0 |
| Microbacterium sp. K2B2 | 0.0 | 0.0 | 4.0 |
| Microbacterium sp. K30 | 3.0 | 16.0 | 3.0 |
| Microbacterium sp. K31 | 2.0 | 8.0 | 4.0 |
| Microbacterium sp. K33 | 2.0 | 16.0 | 3.0 |
| Microbacterium sp. K35 | 2.0 | 24.0 | 1.5 |
| Microbacterium sp. K36 | 3.0 | 24.0 | 1.5 |
| Microbacterium sp. K40 | 3.0 | 12.0 | 3.0 |
| Microbacterium sp. K41 | 1.0 | 1.5 | 1.5 |
| Microbacterium sp. K5D | 1.0 | 24.0 | 4.0 |
| Microbacterium sp. PF3 | 16.0 | 6.0 | 0.1 |
Notes:
Minimum inhibitory concentrations (μg/ml) of ampicillin, chloramphenicol, and vancomycin for each isolate.
Ampicillin (Amp), chloramphenicol (Cam), and vancomycin (Van).
Metal and antibiotic co-tolerance has been reported in a number of environmentally isolated bacteria. Bacteria isolated from drinking water with noted antibiotic resistance were also tolerant to high levels of Cu2+, Pb2+, and Zn2+ (Calomiris, Armstrong & Seidler, 1984). Environmental isolates from wastewater treatment plants have been shown to tolerate various heavy metals and antibiotics (Shafique, Jawaid & Rehman, 2016, 2017). Bacillus spp. isolated from river water tolerated multiple heavy metals and antibiotics (Shammi & Ahmed, 2016). Co-tolerance to heavy metals, antibiotics, and polychlorinated biphenyls was also observed in isolates from Antarctic sediments (Giudice et al., 2013). Further, evidence of shared genetic tolerance mechanisms for metals and antibiotics was found in Staphylococcus aureus isolated from a polluted riverbank. S. aureus contained a novel emrAB operon encoding efflux pumps that were inducible by the heavy metals Cr(VI) and manganese and the antibiotics ampicillin and chloramphenicol (Zhang et al., 2016).
While this study does not prove function, the data suggest the antibiotic tolerances found in these isolates could be related to the antibiotic resistance genes documented in the genomes. For example, 15 out of 16 isolates were able to tolerate chloramphenicol matching the 15 genomes that contained an annotated chloramphenicol resistance gene (cmlR, cmx) (Table S4), a gene previously shown to provide Corynebacterium striatum with chloramphenicol tolerance (Schwarz et al., 2004; Tauch et al., 1998). It is possible cmx could therefore provide tolerance to the Microbacterium isolates. Similarly, all the Microbacterium isolates had an annotated class A beta-lactamase gene (penP), a gene that provides ampicillin tolerance to numerous bacteria (Bush & Jacoby, 2010), though not all isolates were resistant to ampicillin (Table 3).
Implications to community functions
All Microbacterium spp. were isolated from the same contaminated soil and have highly similar 16S rRNA genes (99–100% BLAST identity). Yet, these isolates displayed both genomic variation and varying abilities to tolerate multiple heavy metals and antibiotics. Other studies have shown that closely related isolates can exhibit variable ranges of genomic and physiological differences (Coleman & Chisholm, 2010; Henson et al., 2015; Hunt et al., 2008; Martiny, Coleman & Chisholm, 2006; Meyer & Huber, 2014; Qamar, Rehman & Hasnain, 2017; Rocap et al., 2003; Simmons et al., 2008; Welch et al., 2002). A study of 78 Myxococcus xanthus isolates from a small soil plot found 21 different genotypes (Vos & Velicer, 2006). Thus, it is speculated that genomic and physiological diversity would allow populations to differentiate, potentially increasing resistance and/or resilience that would aid the community to thrive during metals and other contaminant stress. Further support for this could be found when examining the Cr(VI) reduction and resistance data. While none of the isolates had an annotated Cr(VI) resistance efflux pump (chrA), they all contained a Cr(VI) reductase (chrR), yet there was variability found in their ability to reduce and resist Cr(VI). As the isolates from this study were from a soil with multiple contaminants, specific isolates that more efficiently reduce Cr(VI) (making it less bioavailable) may allow other community members (without resistance genes) to thrive and possibly degrade other contaminants. Thus, the variation observed in these isolates could be a reflection of how the environmental stressors (e.g., heavy metals) can promote genomic diversity and a more stable community.
Supplemental Information
Molecular Phylogenetic analysis by Maximum Likelihood method. The tree with the highest log likelihood is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 1181 positions in the final dataset.
MIC (μg/ml) of ampicillin, chloramphenicol, and vancomycin for each isolate.
Acknowledgments
We thank Jorge W. Santo Domingo for assistance with data analysis and helpful comments on the manuscript. We also thank Amber Conley for assistance with lab work. This is contribution number 114 of the CMU Institute for Great Lakes Research.
Funding Statement
Funding was provided by the CMU College of Science and Engineering and the Office of Research and Graduate Studies’ Undergraduate Research and Creative Endeavors grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Deric R. Learman conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Zahra Ahmad performed the experiments, approved the final draft.
Allison Brookshier performed the experiments, approved the final draft.
Michael W. Henson performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Victoria Hewitt performed the experiments, approved the final draft.
Amanda Lis performed the experiments, approved the final draft.
Cody Morrison performed the experiments, approved the final draft.
Autumn Robinson performed the experiments, approved the final draft.
Emily Todaro performed the experiments, approved the final draft.
Ethan Wologo performed the experiments, approved the final draft.
Sydney Wynne performed the experiments, approved the final draft.
Elizabeth W. Alm conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Peter S. Kourtev conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
National Center for Biotechnology Information’s (NCBI) Short Read Archives (SRA), accession number: SRP120551.
Data Availability
The following information was supplied regarding data availability:
The raw data are contained in the Methods section of this article and the Supplemental Materials.
References
- Abou-Shanab, Van Berkum & Angle (2007).Abou-Shanab RAI, Van Berkum P, Angle JS. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68(2):360–367. doi: 10.1016/j.chemosphere.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Ackerley et al. (2004a).Ackerley DF, Gonzalez CF, Keyhan M, Blake R, Matin A. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environmental Microbiology. 2004a;6(8):851–860. doi: 10.1111/j.1462-2920.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- Ackerley et al. (2004b).Ackerley DF, Gonzalez CF, Park CH, Blake R, Keyhan A, Matin A. Chromate-reducing properties of soluble Flavoproteins from Pseudomonas putida and Escherichia coli. Applied and Environmental Microbiology. 2004b;70(2):873–882. doi: 10.1128/Aem.70.2.873-882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul et al. (1997).Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramov et al. (2016).Avramov AP, Couger MB, Hartley EL, Land C, Wellendorf R, Hanafy RA, Budd C, French DP, Hoff WD, Youssef N. Draft genome sequence of Microbacterium oleivorans strain Wellendorf implicates heterotrophic versatility and bioremediation potential. Genomics Data. 2016;10:54–60. doi: 10.1016/j.gdata.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Austin et al. (2006).Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV. Co-selection of antibiotic and metal resistance. Trends in Microbiology. 2006;14(4):176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Barak et al. (2006).Barak Y, Ackerley DF, Dodge CJ, Banwari L, Alex C, Francis AJ, Matin A. Analysis of novel soluble chromate and uranyl reductases and generation of an improved enzyme by directed evolution. Applied and Environmental Microbiology. 2006;72(11):7074–7082. doi: 10.1128/AEM.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett (1991).Bartlett RJ. Chromium cycling in soils and water—links, gaps, and methods. Environmental Health Perspectives. 1991;92:17–24. doi: 10.1289/ehp.919217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard & Pacheco (2015).Bernard K, Pacheco AL. In vitro activity of 22 antimicrobial agents against Corynebacterium and Microbacterium species referred to the Canadian National Microbiology Laboratory. Clinical Microbiology Newsletter. 2015;37(23):187–198. doi: 10.1016/j.clinmicnews.2015.11.003. [DOI] [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann et al. (2010).Bollmann A, Palumbo AV, Lewis K, Epstein SS. Isolation and physiology of bacteria from contaminated subsurface sediments. Applied and Environmental Microbiology. 2010;76(22):7413–7419. doi: 10.1128/Aem.00376-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand et al. (2009).Briand E, Escoffier N, Straub C, Sabart M, Quiblier C, Humbert J-F. Spatiotemporal changes in the genetic diversity of a bloom-forming Microcystis aeruginosa (cyanobacteria) population. ISME Journal. 2009;3(4):419–429. doi: 10.1038/ismej.2008.121. [DOI] [PubMed] [Google Scholar]
- Brose & James (2010).Brose DA, James BR. Oxidation-reduction transformations of chromium in aerobic soils and the role of electron-shuttling quinones. Environmental Science & Technology. 2010;44(24):9438–9444. doi: 10.1021/es101859b. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2012).Brown SD, Palumbo AV, Panikov N, Arlyawansa T, Klingeman DM, Johnson CM, Land ML, Utturkar SM, Epstein SS. Draft genome sequence for Microbacterium laevaniformans strain OR221, a bacterium tolerant to metals, nitrate, and low pH. Journal of Bacteriology. 2012;194(12):3279–3280. doi: 10.1128/JB.00474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. (2006).Brown SD, Thompson MR, Verberkmoes NC, Chourey K, Shah M, Zhou J, Hettich RL, Thompson DK. Molecular dynamics of the Shewanella oneidensis response to chromate stress. Molecular & Cellular Proteomics. 2006;5(6):1054–1071. doi: 10.1074/mcp.M500394-MCP200. [DOI] [PubMed] [Google Scholar]
- Bush & Jacoby (2010).Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrobial Agents and Chemotherapy. 2010;54(3):969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral et al. (2016).Cabral L, Júnior GVL, Pereira de Sousa ST, Dias ACF, Lira Cadete L, Andreote FD, Hess M, de Oliveira VM. Anthropogenic impact on mangrove sediments triggers differential responses in the heavy metals and antibiotic resistomes of microbial communities. Environmental Pollution. 2016;216:460–469. doi: 10.1016/j.envpol.2016.05.078. [DOI] [PubMed] [Google Scholar]
- Calomiris, Armstrong & Seidler (1984).Calomiris JJ, Armstrong JL, Seidler RJ. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Applied and Environmental Microbiology. 1984;47:1238–1242. doi: 10.1128/aem.47.6.1238-1242.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes & Ohtake (1988).Cervantes C, Ohtake H. Plasmid-determined resistance to chromate in Pseudomonas aeruginosa. FEMS Microbiology Letters. 1988;56(2):173–176. doi: 10.1111/j.1574-6968.1988.tb03172.x. [DOI] [Google Scholar]
- Cervantes et al. (1990).Cervantes C, Ohtake H, Chu L, Misra TK, Silver S. Cloning, nucleotide sequence, and expression of the chromate resistance determinant of Pseudomonas aeruginosa plasmid pUM505. Journal of Bacteriology. 1990;172:287–291. doi: 10.1128/jb.172.1.287-291.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan et al. (2013).Chauhan A, Green S, Pathak A, Thomas J, Venkatramanan R. Whole-genome sequences of five oyster-associated bacteria show potential for crude oil hydrocarbon degradation. Genome Announcements. 2013;1:e00802-13. doi: 10.1128/genomeA.00802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Holman & Lin (2012).Cheng Y, Holman H-Y, Lin Z. Remediation of chromium and uranium contamination by microbial activity. Elements. 2012;8:107–112. doi: 10.2113/gselements.8.2.107. [DOI] [Google Scholar]
- Cheng et al. (2009).Cheng Y, Xie Y, Zheng J, Wu Z, Chen Z, Ma X, Li B, Lin Z. Identification and characterization of the chromium(VI) responding protein from a newly isolated Ochrobactrum anthropi CTS-325. Journal of Environmental Sciences-China. 2009;21(12):1673–1678. doi: 10.1016/S1001-0742(08)62472-9. [DOI] [PubMed] [Google Scholar]
- Cheung & Gu (2007).Cheung KH, Gu JD. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. International Biodeterioration & Biodegradation. 2007;59(1):8–15. doi: 10.1016/j.ibiod.2006.05.002. [DOI] [Google Scholar]
- Coleman & Chisholm (2010).Coleman ML, Chisholm SW. Ecosystem-specific selection pressures revealed through comparative population genomics. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18634–18639. doi: 10.1073/pnas.1009480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corretto et al. (2015).Corretto E, Antonielli L, Sessitsch A, Kidd P, Weyens N, Brader G. Draft genome sequences of 10 Microbacterium spp., with emphasis on heavy metal-contaminated environments. Genome Announcements. 2015;3(3):e00432-15. doi: 10.1128/genomeA.00432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decorosi et al. (2009).Decorosi F, Tatti E, Mini A, Giovannetti L, Viti C. Characterization of two genes involved in chromate resistance in a Cr(VI)-hyper-resistant bacterium. Extremophiles: Life Under Extreme Conditions. 2009;13(6):917–923. doi: 10.1007/S00792-009-0279-6. [DOI] [PubMed] [Google Scholar]
- Delmont & Eren (2018).Delmont TO, Eren AM. Linking pangenomes and metagenomes: the Prochlorococcus metapangenome. PeerJ. 2018;6:e4320. doi: 10.7717/peerj.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef et al. (2010).Denef VJ, Kalnejais LH, Mueller RS, Wilmes P, Baker BJ, Thomas BC, VerBerkmoes NC, Hettich RL, Banfield JF. Proteogenomic basis for ecological divergence of closely related bacteria in natural acidophilic microbial communities. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2383–2390. doi: 10.1073/pnas.0907041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy (2011).Eddy SR. Accelerated profile HMM searches. PLOS Computational Biology. 2011;7(10):e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2015).Eren AM, Esen OC, Quince C, Vineis JH, Morrison HG, Sogin ML, Delmont TO. Anvi’o: an advanced analysis and visualization platform for ‘omics data. PeerJ. 2015;3:e1319. doi: 10.7717/peerj.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focardi, Pepi & Focardi (2013).Focardi S, Pepi M, Focardi SE. Microbial reduction of hexavalent chromium as a mechanism of detoxification and possible bioremediation applications. In: Chamy R, Rosenkranz F, editors. Biodegradation—life of science. InTech; 2013. [Google Scholar]
- Frangeul et al. (2008).Frangeul L, Quillardet P, Castets A-M, Humbert J-F, Matthijs HC, Cortez D, Tolonen A, Zhang C-C, Gribaldo S, Kehr J-C, Zilliges Y, Ziemert N, Becker S, Talla E, Latifi A, Billault A, Lepelletier A, Dittmann E, Bouchier C, de Marsac NT. Highly plastic genome of Microcystis aeruginosa PCC 7806, a ubiquitous toxic freshwater cyanobacterium. BMC Genomics. 2008;9(1):274. doi: 10.1186/1471-2164-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh et al. (2000).Ghosh A, Singh A, Ramteke PW, Singh VP. Characterization of large plasmids encoding resistance to toxic heavy metals in Salmonella abortus equi. Biochemical and Biophysical Research Communications. 2000;272(1):6–11. doi: 10.1006/bbrc.2000.2727. [DOI] [PubMed] [Google Scholar]
- Giudice et al. (2013).Giudice AL, Casella P, Bruni V, Michaud L. Response of bacterial isolates from Antarctic shallow sediments towards heavy metals, antibiotics and polychlorinated biphenyls. Ecotoxicology. 2013;22(2):240–250. doi: 10.1007/S10646-012-1020-2. [DOI] [PubMed] [Google Scholar]
- Gneiding, Frodl & Funke (2008).Gneiding K, Frodl R, Funke G. Identities of Microbacterium spp. encountered in human clinical specimens. Journal of Clinical Microbiology. 2008;46(11):3646–3652. doi: 10.1128/JCM.01202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez et al. (2005).Gonzalez CF, Ackerley DF, Lynch SV, Matin A. ChrR, a soluble quinone reductase of Pseudomonas putida that defends against H2O2. Journal of Biological Chemistry. 2005;280(24):22590–22595. doi: 10.1074/jbc.M501654200. [DOI] [PubMed] [Google Scholar]
- Gurevich et al. (2013).Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne et al. (2009a).Henne KL, Nakatsu CH, Thompson DK, Konopka AE. High-level chromate resistance in Arthrobacter sp. strain FB24 requires previously uncharacterized accessory genes. BMC Microbiology. 2009a;9(1):199. doi: 10.1186/1471-2180-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne et al. (2009b).Henne KL, Turse JE, Nicora CD, Lipton MS, Tollaksen SL, Lindberg C, Babnigg G, Giometti CS, Nakatsu CH, Thompson DK, Konopka AE. Global proteomic analysis of the chromate response in Arthrobacter sp. Strain FB24. Journal of Proteome Research. 2009b;8(4):1704–1716. doi: 10.1021/Pr800705f. [DOI] [PubMed] [Google Scholar]
- Henriques et al. (2016).Henriques I, Tacão M, Leite L, Fidalgo C, Araújo S, Oliveira C, Alves A. Co-selection of antibiotic and metal(loid) resistance in gram-negative epiphytic bacteria from contaminated salt marshes. Marine Pollution Bulletin. 2016;109(1):427–434. doi: 10.1016/j.marpolbul.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Henson et al. (2015).Henson MW, Santo Domingo JW, Kourtev PS, Jensen RV, Dunn JA, Learman DR. Metabolic and genomic analysis elucidates strain-level variation in Microbacterium spp. isolated from chromate contaminated sediment. PeerJ. 2015;3:e1395. doi: 10.7717/peerj.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2005).Hu P, Brodie EL, Suzuki Y, McAdams HH, Andersen GL. Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. Journal of Bacteriology. 2005;187(24):8437–8449. doi: 10.1128/JB.187.24.8437-8449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert et al. (2013).Humbert J-F, Barbe V, Latifi A, Gugger M, Calteau A, Coursin T, Lajus A, Castelli V, Oztas S, Samson G, Longin C, Medigue C, de Marsac NT. A tribute to disorder in the genome of the bloom-forming freshwater cyanobacterium Microcystis aeruginosa. PLOS ONE. 2013;8(8):e70747. doi: 10.1371/journal.pone.0070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries et al. (2005).Humphries AC, Nott KP, Hall LD, Macaskie LE. Reduction of Cr(VI) by immobilized cells of Desulfovibrio vulgaris NCIMB 8303 and Microbacterium sp. NCIMB 13776. Biotechnology and Bioengineering. 2005;90(5):589–596. doi: 10.1002/bit.20450. [DOI] [PubMed] [Google Scholar]
- Hunt et al. (2008).Hunt DE, David LA, Gevers D, Preheim SP, Alm EJ, Polz MF. Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science. 2008;320(5879):1081–1085. doi: 10.1126/Science.1157890. [DOI] [PubMed] [Google Scholar]
- Hyatt et al. (2010).Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11(1):119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joynt et al. (2006).Joynt J, Bischoff M, Turco R, Konopka A, Nakatsu CH. Microbial community analysis of soils contaminated with lead, chromium and petroleum hydrocarbons. Microbial Ecology. 2006;51(2):209–219. doi: 10.1007/s00248-005-0205-0. [DOI] [PubMed] [Google Scholar]
- Kettler et al. (2007).Kettler GC, Martiny AC, Huang K, Zucker J, Coleman ML, Rodrigue S, Chen F, Lapidus A, Ferriera S, Johnson J, Steglich C, Church GM, Richardson P, Chisholm SW. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLOS Genetics. 2007;3(12):e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtev, Nakatsu & Konopka (2006).Kourtev PS, Nakatsu CH, Konopka A. Responses of the anaerobic bacterial community to addition of organic C in chromium(VI)- and iron(III)-amended microcosms. Applied and Environmental Microbiology. 2006;72(1):628–637. doi: 10.1128/Aem.72.1.628-637.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtev, Nakatsu & Konopka (2009).Kourtev PS, Nakatsu CH, Konopka A. Inhibition of nitrate reduction by chromium(VI) in anaerobic soil microcosms. Applied and Environmental Microbiology. 2009;75(19):6249–6257. doi: 10.1128/aem.00347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffineur et al. (2003).Laffineur K, Avesani V, Cornu G, Charlier J, Janssens M, Wauters G, Delmée M. Bacteremia due to a novel Microbacterium species in a patient with leukemia and description of Microbacterium paraoxydans sp. nov. Journal of Clinical Microbiology. 2003;41(5):2242–2246. doi: 10.1128/JCM.41.5.2242-2246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016).Li DH, Luo RB, Liu CM, Leung CM, Ting HF, Sadakane K, Yamashita H, Lam TW. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11. doi: 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu ZM, Wu Y, Lei CF, Liu PM, Gao MY. Chromate reduction by a chromate-resistant bacterium, Microbacterium sp. World Journal of Microbiology & Biotechnology. 2012;28(4):1585–1592. doi: 10.1007/s11274-011-0962-5. [DOI] [PubMed] [Google Scholar]
- Manara et al. (2012).Manara A, DalCorso G, Baliardini C, Farinati S, Cecconi D, Furini A. Pseudomonas putida response to cadmium: changes in membrane and cytosolic proteomes. Journal of Proteome Research. 2012;11(8):4169–4179. doi: 10.1021/pr300281f. [DOI] [PubMed] [Google Scholar]
- Markowitz et al. (2012).Markowitz VM, Chen IMA, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, Jacob B, Huang JH, Williams P, Huntemann M, Anderson I, Mavromatis K, Ivanova NN, Kyrpides NC. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Research. 2012;40(D1):D115–D122. doi: 10.1093/Nar/Gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny, Coleman & Chisholm (2006).Martiny AC, Coleman ML, Chisholm SW. Phosphate acquisition genes in Prochlorococcus ecotypes: evidence for genome-wide adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12552–12557. doi: 10.1073/pnas.0601301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney, McNally & O’Connell (2017).McInerney JO, McNally A, O’Connell MJ. Why prokaryotes have pangenomes. Nature Microbiology. 2017;2(4):17040. doi: 10.1038/nmicrobiol.2017.40. [DOI] [PubMed] [Google Scholar]
- Meyer et al. (2017).Meyer KA, Davis TW, Watson SB, Denef VJ, Berry MA, Dick GJ. Genome sequences of lower Great Lakes Microcystis sp. reveal strain-specific genes that are present and expressed in western Lake Erie blooms. PLOS ONE. 2017;12(10):e0183859. doi: 10.1371/journal.pone.0183859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer & Huber (2014).Meyer JL, Huber JA. Strain-level genomic variation in natural populations of Lebetimonas from an erupting deep-sea volcano. ISME Journal. 2014;8(4):867–880. doi: 10.1038/ismej.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda et al. (2005).Miranda AT, González MV, González G, Vargas E, Campos-Garcia J, Cervantes C. Involvement of DNA helicases in chromate resistance by Pseudomonas aeruginosa PAO1. Mutation Research. 2005;578(1–2):202–209. doi: 10.1016/j.mrfmmm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Nakatsu et al. (2005).Nakatsu CH, Carmosini N, Baldwin B, Beasley F, Kourtev P, Konopka A. Soil microbial community responses to additions of organic carbon substrates and heavy metals (Pb and Cr) Applied and Environmental Microbiology. 2005;71(12):7679–7689. doi: 10.1128/Aem.71.12.7679-7689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelkova et al. (2007).Nedelkova M, Merroun ML, Rossberg A, Hennig C, Pobell SS. Microbacterium isolates from the vicinity of a radioactive waste depository and their interactions with uranium. FEMS Microbiology Ecology. 2007;59(3):694–705. doi: 10.1111/j.1574-6941.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Nies (1995).Nies DH. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. Journal of Bacteriology. 1995;177(10):2707–2712. doi: 10.1128/jb.177.10.2707-2712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies (2003).Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiology Reviews. 2003;27(2–3):313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Nies, Nies & Silver (1990).Nies A, Nies DH, Silver S. Nucleotide sequence and expression of a plasmid-encoded chromate resistance determinant from Alcaligenes eutrophus. Journal of Biological Chemistry. 1990;265:5648–5653. [PubMed] [Google Scholar]
- Niewerth et al. (2012).Niewerth H, Schuldes J, Parschat K, Kiefer P, Vorholt JA, Daniel R, Fetzner S. Complete genome sequence and metabolic potential of the quinaldine-degrading bacterium Arthrobacter sp. Rue61a. BMC Genomics. 2012;13(1):534. doi: 10.1186/1471-2164-13-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman, Piater & van Heerden (2008).Opperman DJ, Piater LA, van Heerden E. A novel chromate reductase from Thermus scotoductus SA-01 related to old yellow enzyme. Journal of Bacteriology. 2008;190(8):3076–3082. doi: 10.1128/Jb.01766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaktas, Taskin & Gozen (2012).Ozaktas T, Taskin B, Gozen AG. High level multiple antibiotic resistance among fish surface associated bacterial populations in non-aquaculture freshwater environment. Water Research. 2012;46(19):6382–6390. doi: 10.1016/j.watres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Oze et al. (2004).Oze C, Fendorf S, Bird DK, Coleman RG. Chromium geochemistry in serpentinized ultramafic rocks and serpentine soils from the Franciscan Complex of California. American Journal of Science. 2004;304(1):67–101. doi: 10.2475/ajs.304.1.67. [DOI] [Google Scholar]
- Park et al. (2000).Park CH, Keyhan M, Wielinga B, Fendorf S, Matin A. Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Applied and Environmental Microbiology. 2000;66(5):1788–1795. doi: 10.1128/aem.66.5.1788-1795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks et al. (2015).Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Research. 2015;25(7):1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanapipitpaisal, Brown & Macaskie (2001).Pattanapipitpaisal P, Brown NL, Macaskie LE. Chromate reduction by Microbacterium liquefaciens immobilised in polyvinyl alcohol. Biotechnology Letters. 2001;23(1):61–65. doi: 10.1023/A:1026750810580. [DOI] [Google Scholar]
- Qamar, Rehman & Hasnain (2017).Qamar N, Rehman Y, Hasnain S. Arsenic-resistant and plant growth-promoting Firmicutes and γ-Proteobacteria species from industrially polluted irrigation water and corresponding cropland. Journal of Applied Microbiology. 2017;123(3):748–758. doi: 10.1111/jam.13535. [DOI] [PubMed] [Google Scholar]
- Ramirez-Diaz et al. (2008).Ramirez-Diaz MI, Diaz-Perez C, Vargas E, Riveros-Rosas H, Campos-Garcia J, Cervantes C. Mechanisms of bacterial resistance to chromium compounds. Biometals. 2008;21(3):321–332. doi: 10.1007/s10534-007-9121-8. [DOI] [PubMed] [Google Scholar]
- Rocap et al. (2003).Rocap G, Larimer FW, Lamerdin J, Malfatti S, Chain P, Ahlgren NA, Arellano A, Coleman M, Hauser L, Hess WR, Johnson ZI, Land M, Lindell D, Post AF, Regala W, Shah M, Shaw SL, Steglich C, Sullivan MB, Ting CS, Tolonen A, Webb EA, Zinser ER, Chisholm SW. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature. 2003;424(6952):1042–1047. doi: 10.1038/Nature01947. [DOI] [PubMed] [Google Scholar]
- Schwarz et al. (2004).Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiology Reviews. 2004;28(5):519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Seiler & Berendonk (2012).Seiler C, Berendonk TU. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Frontiers in Microbiology. 2012;3:399. doi: 10.3389/fmicb.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafique, Jawaid & Rehman (2016).Shafique M, Jawaid A, Rehman Y. As(V) Reduction, As(III) oxidation, and Cr(VI) reduction by multi-metal-resistant Bacillus subtilis, Bacillus safensis, and Bacillus cereus species isolated from wastewater treatment plant. Geomicrobiology Journal. 2016;34(8):687–694. doi: 10.1080/01490451.2016.1240265. [DOI] [Google Scholar]
- Shafique, Jawaid & Rehman (2017).Shafique M, Jawaid A, Rehman Y. Redox biotransformation of arsenic along with plant growth promotion by multi-metal resistance Pseudomonas sp. MX6. Comptes Rendus Biologies. 2017;340(6–7):330–338. doi: 10.1016/j.crvi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Shammi & Ahmed (2016).Shammi T, Ahmed S. Heavy metal tolerance and antibiotic resistance of Bacillus spp. isolated from two major rivers in Bangladesh. Bangladesh Journal of Microbiology. 2016;30(1–2):17–22. doi: 10.3329/bjm.v30i1-2.28448. [DOI] [Google Scholar]
- Silver (1996).Silver S. Bacterial resistances to toxic metal ions—a review. Gene. 1996;179(1):9–19. doi: 10.1016/S0378-1119(96)00323-X. [DOI] [PubMed] [Google Scholar]
- Simmons et al. (2008).Simmons SL, DiBartolo G, Denef VJ, Goltsman DSA, Thelen MP, Banfield JF. Population genomic analysis of strain variation in Leptospirillum Group II bacteria involved in acid mine drainage formation. PLOS Biology. 2008;6(7):e177. doi: 10.1371/journal.pbio.0060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni et al. (2014).Soni SK, Singh R, Awasthi A, Kalra A. A Cr(VI)-reducing Microbacterium sp. strain SUCR140 enhances growth and yield of Zea mays in Cr(VI) amended soil through reduced chromium toxicity and improves colonization of arbuscular mycorrhizal fungi. Environmental Science and Pollution Research. 2014;21(3):1971–1979. doi: 10.1007/s11356-013-2098-7. [DOI] [PubMed] [Google Scholar]
- Tamura & Nei (1993).Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tauch et al. (1998).Tauch A, Zheng Z, Pühler A, Kalinowski J. Corynebacterium striatum chloramphenicol resistance transposon Tn5564:Genetic organization and transposition in Corynebacterium glutamicum. Plasmid. 1998;40(2):126–139. doi: 10.1006/plas.1998.1362. [DOI] [PubMed] [Google Scholar]
- Ug & Ceylan (2003).Ug A, Ceylan Ö. Occurrence of resistance to antibiotics, metals, and plasmids in clinical strains of staphylococcus spp. Archives of Medical Research. 2003;34(2):130–136. doi: 10.1016/S0188-4409(03)00006-7. [DOI] [PubMed] [Google Scholar]
- Urone (1955).Urone PF. Stability of colorimetric reagent for chromium, s-Diphenylcarbazide, in various solvents. Division of Industrial Hygiene. 1955;27(8):1354–1355. doi: 10.1021/ac60104a048. [DOI] [Google Scholar]
- Viti et al. (2013).Viti C, Marchi E, Decorosi F, Giovannetti L. Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiology Reviews. 2013;38(4):633–659. doi: 10.1111/1574-6976.12051. [DOI] [PubMed] [Google Scholar]
- Vos & Velicer (2006).Vos M, Velicer GJ. Genetic population structure of the soil bacterium Myxococcus xanthus at the centimeter scale. Applied and Environmental Microbiology. 2006;72(5):3615–3625. doi: 10.1128/AEM.72.5.3615-3625.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang H, Xiang T, Wang Y, Song J, Zhai Y, Chen X, Li Y, Zhao B, Zhao B, Ruan Z. Microbacterium petrolearium sp. nov., isolated from an oil-contaminated water sample. International Journal of Systematic and Evolutionary Microbiology. 2014;64(Pt 12):4168–4172. doi: 10.1099/ijs.0.061119-0. [DOI] [PubMed] [Google Scholar]
- Welch et al. (2002).Welch RA, Burland V, Plunkett G, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HLT, Donnenberg MS, Blattner FR. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright et al. (2006).Wright MS, Peltier GL, Stepanauskas R, McArthur JV. Bacterial tolerances to metals and antibiotics in metal-contaminated and reference streams. FEMS Microbiology Ecology. 2006;58(2):293–302. doi: 10.1111/j.1574-6941.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang H, Ma Y, Liu P, Li X. Multidrug resistance operon emrAB contributes for chromate and ampicillin co-resistance in a Staphylococcus strain isolated from refinery polluted river bank. SpringerPlus. 2016;5(1):1648. doi: 10.1186/s40064-016-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular Phylogenetic analysis by Maximum Likelihood method. The tree with the highest log likelihood is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 1181 positions in the final dataset.
MIC (μg/ml) of ampicillin, chloramphenicol, and vancomycin for each isolate.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are contained in the Methods section of this article and the Supplemental Materials.