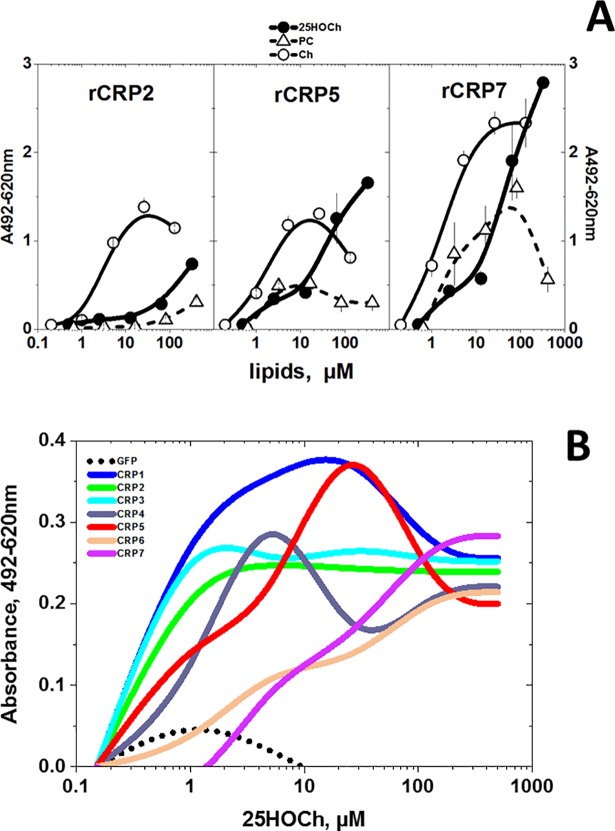
Fig 3.
rCRP (A) and ssCRP1-7 (B) binding to solid-phase lipids. The binding of purified rCRPs and ssCRP1-7 to selected lipids was assayed by using 96-well plates coated to dryness with several lipid concentrations dissolved in ethanol. The lipid-coated plates were washed and were incubated with rCRP2 or ssCRP1-7 in borate buffer for 1 h in a 50 μl volume. To detect bound rCRP or ssCRP1-7, rabbit anti-CRP p3 peptide, peroxidase-labeled goat anti-rabbit IgG and OPD were used as described previously [37,38]. The means and standard deviation from 2 independent experiments were represented. A) rCRP at 0.5 μg/well in borate buffer. Open triangles, solid-phase phosphatidylcholine (PC). Open circles, solid-phase Ch. Black circles, solid-phase 25HOCh. B) ssCRP1-7 were 10-fold diluted in borate buffer. Results from one experiment out of three were interpolated and smoothed using the cubic B-spline method in Origin Pro 2017 (Northampton, MA, USA) (see data in S4 Table). Black points, supernatant from pMCV1.4-gfp transfected cells. Blue line, supernatant from pMCV1.4-crp1 transfected cells. Green line, supernatant from pMCV1.4-crp2 transfected cells. Light-blue line, supernatant from pMCV1.4-crp3 transfected cells. Gray line, supernatant from pMCV1.4-crp4 transfected cells. Red line, supernatant from pMCV1.4-crp5 transfected cells. Orange line, supernatant from pMCV1.4-crp6 transfected cells. Purple line, supernatant from pMCV1.4-crp7 transfected cells.