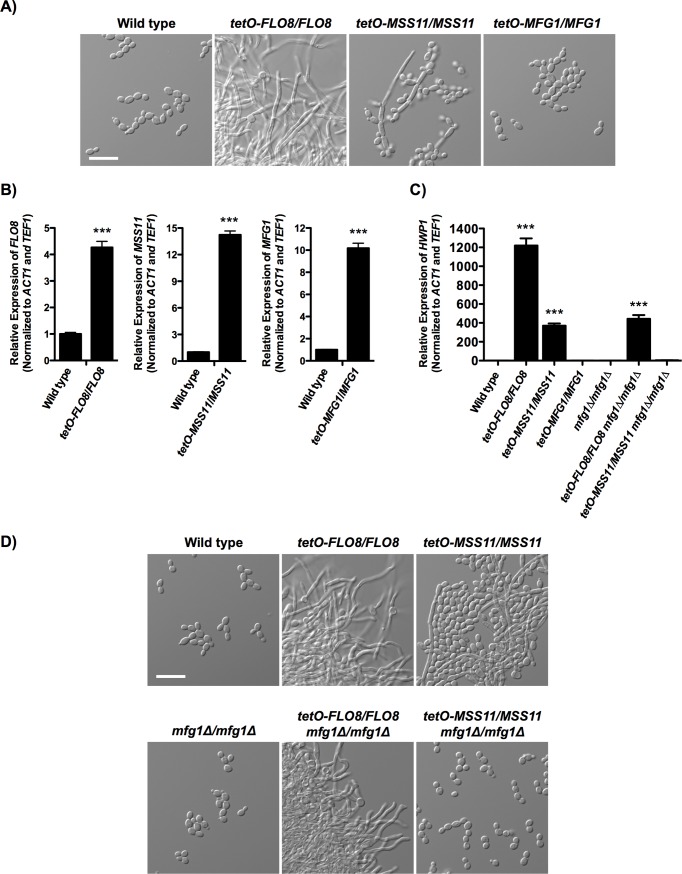
Fig 2. Overexpression of FLO8 or MSS11 results in filamentous growth in the absence of an inducing cue, but overexpression of MFG1 does not.
A) FLO8, MSS11 or MFG1 were overexpressed by replacing the native promoter of one allele with a tetracycline-repressible promoter, tetO. Cells were grown in YPD at 30°C for 6 hours. Scale bar is 20 μm. B) Individual overexpression of genes encoding each complex member was achieved by replacing the native promoter of one allele with a tetracycline-repressible promoter, tetO, which in the absence of tetracycline drives constitutive expression of the target gene. Cells were grown in YPD at 30°C for 3 hours. Transcript levels were normalized to ACT1 and TEF1 and error bars represent standard error of technical triplicates. Assays were performed in biological duplicate. Asterisks indicate P< 0.0001 (***) relative to the wild type (two tailed unpaired t-test). C) Filamentation was quantified by monitoring expression of the filament-specific transcript HWP1 using qRT-PCR, and normalizing to ACT1 and TEF1. Cells were grown for 4 hours in YPD at 30°C. Error bars represent standard error of technical triplicates as is representative of two biological replicates. Asterisks indicate P < 0.0001 (***) relative to parental strain (one-way ANOVA, Bonferroni Multiple Comparison Test). D) Filamentation induced by overexpression of FLO8 is largely independent of Mfg1, whereas filamentation induced by overexpression of MSS11 is completely dependent on Mfg1. Cells were grown in YPD at 30°C for 6 hours. Scale bar is 20 μm.