Abstract
Background
Ensemble recording methods are pervasive in basic and clinical neuroscience research. Invasive neural implants are used in patients with drug resistant epilepsy to localize seizure origin, in neuropsychiatric or Parkinson’s patients to alleviate symptoms via deep brain simulation (DBS), and with animal models to conduct basic research. Studies addressing the brain’s physiological response to chronic electrode implants demonstrate that the mechanical trauma of insertion is followed by an acute inflammatory response as well as a chronic foreign body response. Despite use of invasive recording methods with animal models and humans, little is known of their effect on behavior in healthy populations.
Objective
To quantify the effect of chronic electrode implantation targeting the hippocampus on recognition memory performance.
Methods
Four healthy female rhesus macaques were tested in a delayed nonmatching-to-sample (DNMS) recognition memory task before and after hippocampal implantation with a tetrode array device.
Results
Trials to criterion and recognition memory performance were not significantly different before versus after chronic electrode implantation.
Conclusion
Our results suggest that chronic implants did not produce significant impairments on DNMS performance.
Introduction
Since 1875, when Richard Caton conducted the first neural recordings, electrophysiology and neural implants have played a critical role in our understanding of the brain. Today, neural implants continue to be at the forefront of systems neuroscience research, including high-density electrode arrays, silicon probes, fiber-optic or “optoelectrodes”, and hyperdrive implants with moveable electrodes providing access for recording or stimulating brain tissue.1 Recently, this technology has begun to move into the clinic, where deep brain stimulation (DBS) has been approved by the FDA for treatment of Parkinson’s disease, essential tremor, dystonia, and obsessive compulsive disorder (OCD), and is being investigated as a potential treatment to a number of other diseases and disorders.2 One concern of using electrode technology however, is tissue damage and a subsequent foreign body response to chronic electrodes.
Fortunately, the brain’s response to these neural implants has been well studied. Typically, probe insertion can be characterized by immediate damage to neurons, glia, and capillaries in the path of the electrode, resulting in microhemorrhages as well as activation and migration of microglia.3 Over time, prolonged inflammation at the interface between the tissue and foreign body continues to elicit reactive astrocytes, which make a glial scar in the form of a 25-600 μm sheath surrounding the implant, as well as reactive microglia.4–5 It is currently unclear, however, what factors dictate whether nearby neurons die or simply migrate away from the implant, but both responses have been characterized.4–5 Although it is known that neural implants damage tissue and trigger a long-term immune response, it is unclear whether these effects are large enough to affect cognition.
Here, we investigate the effect on recognition memory of chronically implanted hyperdrive devices carrying 12 tetrode recording probes targeting the hippocampus. We use the delayed nonmatching-to-sample (DNMS) task, a sensitive measure of visual recognition memory that lesion studies suggest relies on the dorsolateral prefrontal cortex, perirhinal cortex, and the hippocampus.6–7 Paradoxically, it has been observed that small lesions in the hippocampus can produce large DNMS behavioral deficits, while, larger lesions result in performance levels closer to those of controls, presumably facilitating the use of alternative brain systems capable of supporting DNMS behavior.7 Observations of behavioral facilitation after damaging the hippocampus are not uncommon,8 and have been attributed to the presence of multiple interacting memory systems.8 The inverse correlation uncovered by Baxter and Murray7 is unique, however, suggesting that some small level of hippocampal damage may significantly disrupt the function of circuits supporting DNMS performance. Their finding suggests the possibility that hippocampal damage caused by electrodes could negatively affect DNMS performance. Therefore, the experiment conducted here represents an important test of the effect of electrode implants on memory.
Methods
Subjects
Four female rhesus macaques (age 7, 10, 14, and 26 years) completed a first round of DNMS testing. After testing, each animal underwent right hemisphere hyperdrive implantation (right hemisphere total implant time 8, 123, 122, and 53 days), removal of right hemisphere electrodes, left hemisphere implantation (158, 222, 165, and 135 days), and then completed a second round of DNMS testing with left hemisphere electrodes intact (at age 15, 16, 18, and 31 years, respectively). Due to surgery-related complications, one animal underwent multiple implants on each hemisphere. Figure 1 shows the procedural timeline for each animal. Due to the large range of age at testing (7-31 years), and varying time between test and retest (4-7.5 years), we also employed a large sample of intact control animals (N=91) aged 3 to 32 years, who completed one round of DNMS testing. The distribution of ages in the control group was bimodal, with a young group ranging in age from 3-14 years (N=34, 22 female, mean: 14.11, std: 3.04) and aged group ranging in age from 20-32 years (N=57, 37 female, mean: 25.09, std: 3.15). All procedures were in accordance with the University of California, Davis Institutional Animal Care and Use Committee.
Figure 1. Procedural timeline.
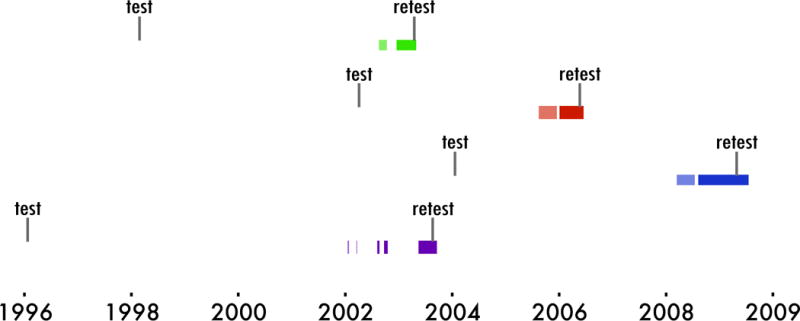
The date of testing and retesting is indicated relative to implantation. Lightly shaded colors represent the period of right hemisphere implantation, and darker colors represent the period of left hemisphere implantation. Retesting was completed with left hemisphere electrodes intact.
DNMS
Delayed nonmatching-to-sample testing was conducted as previously reported.9–10 Briefly, each trial began with a sample phase, wherein a single object was presented along with a food reward. Next, the stimulus was hidden for a variable delay interval period of 10, 15, 60 or 120 seconds. Then, the original object was presented along with a novel object, which covered another food reward, and the correct response is to displace the novel (“nonmatch”) object. Each animal completed 20 trials per day, 5 days a week. Initially each animal was trained to criterion at a 10 second delay. After a cumulative accuracy of 90% calculated across 5 days was achieved, 5 days of testing were conducted at 15, 60, and 120 second delay intervals. Delayed response11 and object discrimination12 testing was also performed at the same time as pre-surgical DNMS testing. This data is reported in Table S1.
Hyperdrive implant
Procedures and specifications of the implanted hyperdrives are described in detail in.13–14 Briefly, the body of the hippocampus was targeted to plate 68 of the Paxinos atlas15 using pre-operative structural MRI. Each hyperdrive consisted of 12 independently movable tetrodes, a reference electrode, and a ground electrode. Each movable electrode consisted of 28-gauge (320 μm) stainless steel guide cannulae, which extended from the top of the skull to a target location, 1-2 mm above the hippocampus. From the base of the guide cannulae, 160 μm silica tubing encasing tetrodes constructed with 30μm polyimide-coated nichrome wire, were lowered through the entirety of the hippocampus.
Quantile regression
In order to better distinguish between the effects of aging and those of the implants on DNMS behavior, we secured a larger cross-sectional dataset from which to estimate the declines in DNMS performance with aging. With this dataset, we employed quantile regression, a variant of linear regression which fits unique estimates for each proportion or quantile of data. A quantile regression at the 50th percentile corresponds to fitting the median. Estimates were calculated from each percentile from 1 to 99 independently for trials to criterion, performance at each delay interval, and average performance across delay intervals. The performance measure of each experimental animal was then converted to a percentile using nearest neighbor interpolation yielding a graded performance metric (1st to 99th percentile) relative to the scores of their peers of the same age. K-fold and leave one out cross-validation were used to determine that a 2nd order model was appropriate for the trials to criterion measure and 1st order models were appropriate for DNMS accuracy, although we note that statistics were performed with either 1st or 2nd order model fits and our results were not sensitive to model order. Analysis was performed using Python’s (Python Software Foundation, Wilmington, Delaware) StatsModels package.
Statistical tests
Repeated measures factorial ANOVAs with testing session (pre- versus post-implant) and delay interval (15, 60, and 120 seconds) as factors were computed on raw and quantile-corrected performance scores. Additionally, pairwise Mann-Whitney U tests compared pre- to post-implant scores for both raw and quantile corrected measures using Python’s SciPy module. P values for pairwise tests were corrected with the Bonferroni correction, where 5 tests were considered (trials to criterion, performance at 15, 60, and 120 second delays, and average performance across delays).
Volume loss estimation
To calculate an estimation of hippocampal volume loss, we quantified percent volume loss in the right hemisphere hippocampus for the two subjects which demonstrated the least and most hippocampal damage respectively. Hippocampal volume loss due to implantation was conducted by comparing whole hippocampal volume calculated from pre-surgical structural MRI with post-surgical volume calculated from post-mortem Nissl-stained sections. Pre-surgical whole hippocampal volume was calculated using methods and data reported in Shamy et al.16 Post-surgical hippocampal volume was calculated by locating regions of the hippocampus on Nissl-stained sections using the demarcation protocol reported by Kyle et al.17 To account for volume decreases that take place during the fixation process, histology-based volume was multiplied by a correction factor of 1.331 (mm3/mm3) or 1.1 cubed to account for the 10% reduction of tissue size in each in each x,y,z dimension that was observed by Kyle et al.17
Results
Percent hippocampal volume loss was estimated in the right hemisphere for two subjects, who, by visual inspection showed the least extensive and most extensive damage respectively. The animal with the least hippocampal damage displayed a reduction in volume of 7% (green data throughout), while the animal with the most displayed a reduction in volume of 24% (purple data throughout). Unfortunately it is unclear how percent volume loss due to ibotenic acid lesions and frank cell death compares with volume loss due to electrode implantation, as the volume loss in the latter could result from cell loss, but also could result from other factors such as compression of the extracellular space. Regardless of the reason for the volume loss, the percent loss was, at least superficially, similar between our data and the small lesion group of Baxter and Murray7.
Trials to criterion and performance at 15, 60, and 120 seconds were assessed before and after hyperdrive implant (Figure 2). A 2x3 session (test vs. retest) by delay interval repeated measures ANOVA revealed a main effect of delay interval (F(2,8) = 8.023, p = 0.02) but did not reveal an effect of test versus retest or an interaction (p>0.14). Furthermore, planned comparisons of pre- versus post-implant metrics were conducted on trials to criterion, performance at each delay interval, and average performance across delay intervals using Mann-Whitney U-tests. All tests failed to pass a significance threshold of p=0.05 (all p> .09), and the test with the lowest p-value showed a numerically, but not significantly better performance at retest for the 15 second delay interval. We also note that paired t-tests revealed a similar pattern of results with no comparisons reaching statistical significance. An a priori power analysis was conducted based on data from Beason-Held et al.18, which is the source of the animals with the smallest lesions and largest deficits in the meta-analysis of Baxter and Murray7. This analysis suggested that 4 subjects would have 89% power to detect the 16-point deficit seen by Beason-Held et al.18 in DNMS accuracy. Thus, if a decline in performance of this magnitude was present, we should have detected it. While our analysis suggests that hyperdrive implant did not significantly affect DNMS accuracy, this analysis did not control for age-related changes in DNMS performance. Variability in DNMS performance has been shown to increase significantly with age,19 which may have affected our ability to detect a change in our sample. Therefore, we conducted quantile regression to better control for these factors (Figure 3, see methods for analysis details).
Figure 2. Raw DNMS performance.
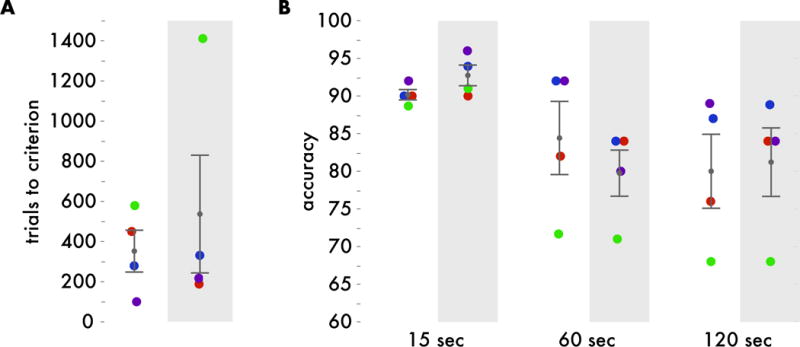
A. Number of trials performed before reaching performance criteria before implant (white background) and after implant (gray background). B. Recognition performance (percent correct) at 15, 60, and 120 second delay intervals before implant (white background) and after implant (gray background). Ages of each animal at test and retest are consistent throughout all figures – with the ages at original test being: purple 7 years, blue 10 years, red 14 years, and green 26 years.
Figure 3. Correcting for age-related performance changes.
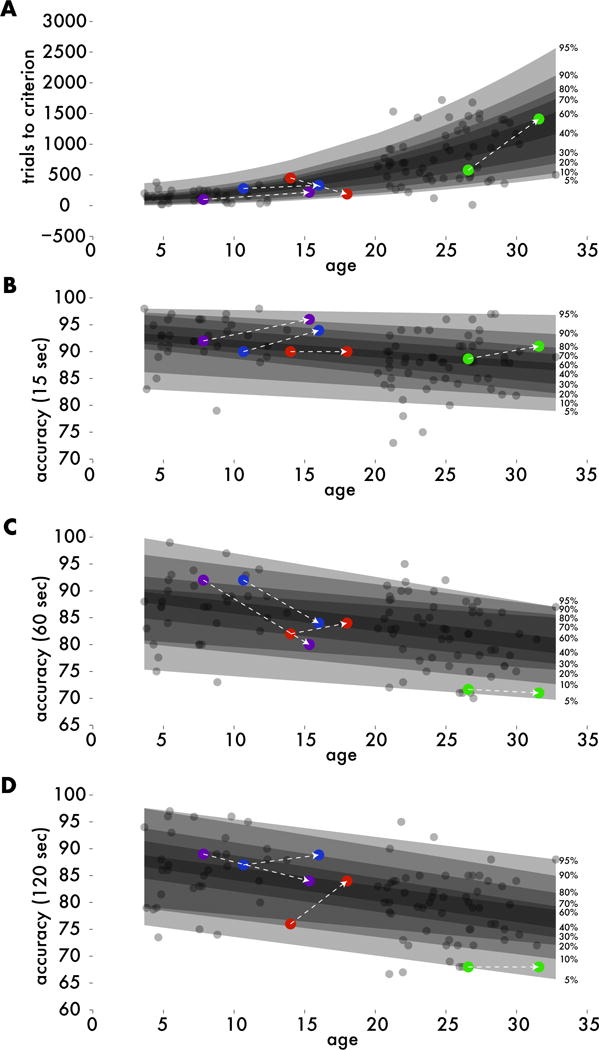
Quantile regression estimated the unique distribution of performance scores across the rhesus lifespan (transparent patches). Each experimental animal’s (colored dots) raw performance metrics were converted to a relative percentile compared to age-matched controls (gray dots). A. Number of trials performed before reaching performance criteria before implant was best fit with a second-order model. B-D. Recognition performance (percent correct) at 15, 60, and 120 second delay intervals were best fit with first-order models.
Converting each experimental test score, to an age-corrected percentile and repeating the same statistical tests revealed a similar pattern of results (Figure 4). Because performance metrics now reflected performance relative to age matched controls, the main effect of delay interval, as expected, was no longer significant (p=.34). Additionally, effects of test versus retest and interactions also remained nonsignificant (p>.09). Pairwise Mann-Whitney U-tests revealed that performance at the fifteen second delay interval increased at retest (p=0.03, uncorrected), however uncorrected p-values are inappropriate for this comparison and the effect does not pass a .05 alpha level when applying the Bonferroni correction (p=.15, corrected). Pairwise comparisons for performance at sixty second, one hundred twenty second, and average across delay intervals were nonsignificant (p>.2, uncorrected). Overall, this suggests that hyperdrive implant does not significantly affect DNMS performance in healthy rhesus macaques.
Figure 4. Percentile corrected DNMS performance.
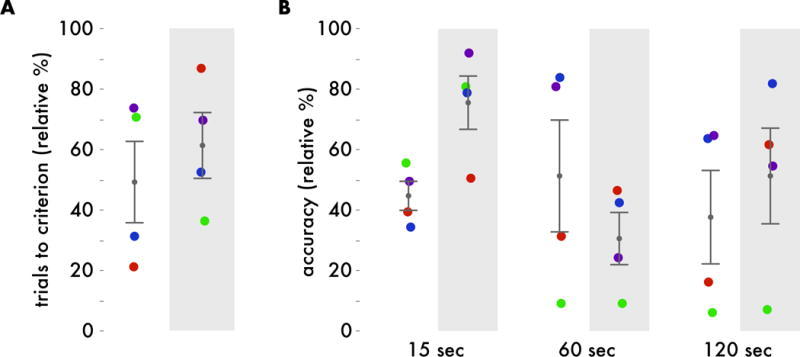
A. Age-matched percentile of number of trials performed before reaching performance criteria before implant (white background) and after implant (gray background). Higher percentiles indicate lower performance. B. Age-matched percentile of recognition performance (percent correct) at 15, 60, and 120 second delay intervals before implant (white background) and after implant (gray background). Higher percentiles indicate better performance.
Discussion
The results of the present analysis suggest that DNMS performance was not significantly affected by chronic electrode implants targeting the hippocampus in healthy rhesus macaques. This indicates that the tissue damage and subsequent foreign body response caused by hyperdrive implant was not sufficient to disrupt hippocampal circuits and impair recognition memory performance to the same degree as the small lesions reported in Baxter and Murray.7
Another important aspect of our study is that no electrical stimulation of brain regions was conducted, making our data of particular interest to research into clinical DBS, where the effects of chronic electrode implantation are confounded with the effects of the electrical stimulation and potential interactions due to the use of patient populations. Our data complement findings that suggest chronic electrode implantation paired with electrical stimulation typically result in minor, if any, long-term cognitive side effects. For instance, an investigation comparing bilateral DBS of basal ganglia structures compared to non-invasive medical therapy showed small, yet significant, decreases in measures of working memory and processing speed.20 A meta-analysis summarizing the work on subthalamic nucleus DBS to treat Parkinson’s, however, concluded that “cognitive and psychiatric effects are relatively rare”.21 In the hippocampus, stimulation as a treatment of temporal lobe epilepsy was found to cause no significant difference in neuropsychological examinations before versus after implantation and stimulation.22 Additionally, stimulation of the subgenual cingulate in subjects with treatment-resistant depression showed no adverse cognitive effects and some improvements on frontal cortical dependent tasks.23 In each of these cases, the cognitive outcomes could not be disentangled from the use of neural stimulation. It is worth noting that the form factor of electrodes in surgical applications are typically non-moveable macro-electrodes, admittedly with much larger profiles than the tetrode micro-electrodes that were slowly lowered (10s of microns at a time) over days. The guide cannulae that assure accurate electrode placements in deep temporal lobe structures, however, were larger. Admittedly, these differences in electrode configuration limits the ability to directly compare results between these studies in monkeys and those in humans, taken together, our study and the clinical DBS literature appear to indicate that behavior is typically resilient to the disturbances caused by chronically implanted electrodes.
Another caveat with the current data is that although we find no differences in performance pre- versus post-implantation, our control group did not undergo a second round of DNMS testing and therefore, it is possible that a control group would have performed better at retest than first test. Thus, one interpretation of our data is that chronic implants left behavior intact but may have blocked improvements that may otherwise have resulted from additional practice. Arguing against this interpretation, a previous study observed no improvement in accuracy in their control groups at re-test compared to the original test.24 This suggests that DNMS performance is relatively resistant to practice effects. Another limitation is that different age groups could be differentially affected by implantation. Our experimental animals were sampled sparsely across the full age range of the rhesus macaque, and thus, we cannot rule out that samples at other ages than examined here might show differing resilience to electrode implantation. Additionally, because the focus of this study was healthy populations, this study does not speak to whether patient populations may experience cognitive side effects due to chronic electrode implantation. In the clinical literature, DBS has been shown to produce little or no cognitive side effects across a wide age range; however, each age group was tested in different patient populations, with different brain regions targeted. Finally, our data were somewhat underpowered to detect very small changes in DNMS performance. A power analysis, however, suggested that we should be able to detect changes on the order of ~15 percentage points.
Conclusion
The present study uses measures of DNMS performance to assess whether chronic neural recording devices affect recognition memory performance. Our results indicate that memory performance is not impaired after implantation, suggesting that the initial tissue damage and subsequent foreign body response caused by hyperdrive implant was not sufficient to disrupt hippocampal circuits and impair recognition memory performance. Overall, the current data support clinical trial data suggesting that long-term cognitive side effects are relatively rare. There currently exists a wide assortment of form factors of neural implants. Our results suggest that deep, dispersed (~0.5 mm between individual electrodes) implants containing tens of electrodes do not significantly disrupt critical brain circuits for recognition memory. As the applications and technologies of neural implants continue to advance, further work will be needed in order to understand the potential impact on memory.
Supplementary Material
Acknowledgments
Supported by: McKnight Brain Research Foundation, NIH grants R01 AG003376, P51 RR000169, R01 NS076856, AG10606, and in part by the Intramural Research Program of the National Institute on Aging.
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Authorship Statement: Carol Barnes conceived and oversaw the study. Peter Rapp oversaw all behavioral testing and provided large cross-sectional data set. Michele Permenter and Julie Vogt prepared data and cared for the experimental animals. Colin Kyle analyzed data with input from Carol Barnes and Peter Rapp. All authors approved the final manuscript.
References
- 1.Buzsaki G, et al. Tools for probing local circuits: high-density silicon probes combined with optogenetics. Neuron. 2015;86:92–105. doi: 10.1016/j.neuron.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariz M, Blomstedt P, Zrinzo L. Future of brain stimulation: new targets, new indications, new technology. Mov Disord. 2013;28:1784–1792. doi: 10.1002/mds.25665. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez E, et al. Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects. Front Neuroeng. 2014;7:24. doi: 10.3389/fneng.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Shen W, et al. Extracellular matrix-based intracortical microelectrodes: Toward a microfabricated neural interface based on natural materials. Microsystems & Nanoengineering. 2015;1:15010. doi: 10.1038/micronano.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Impairment in delayed nonmatching to sample following lesions of dorsal prefrontal cortex. Behav Neurosci. 2012;126:772–780. doi: 10.1037/a0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Kim JJ, Baxter MG. Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends Neurosci. 2001;24:324–330. doi: 10.1016/s0166-2236(00)01818-x. [DOI] [PubMed] [Google Scholar]
- 9.Thome A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016;21:1257–1262. doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160:300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- 12.Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- 13.Thome A, Erickson CA, Lipa P, Barnes CA. Differential effects of experience on tuning properties of macaque MTL neurons in a passive viewing task. Hippocampus. 2012;22:2000–2011. doi: 10.1002/hipo.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skaggs WE, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos, Huang, Toga . The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1999. [Google Scholar]
- 16.Shamy JL, et al. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Kyle CT, et al. Cytoarchitectonically-driven MRI atlas of nonhuman primate hippocampus: Preservation of subfield volumes in aging. Hippocampus. 2017 doi: 10.1002/hipo.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12:481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- 20.Weaver FM, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 22.Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66:1490–1494. doi: 10.1212/01.wnl.0000209300.49308.8f. [DOI] [PubMed] [Google Scholar]
- 23.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Zola-Morgan S, Squire LR, Amaral DG. Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce long-lasting memory impairment in monkeys. J Neurosci. 1989;9:898–913. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


