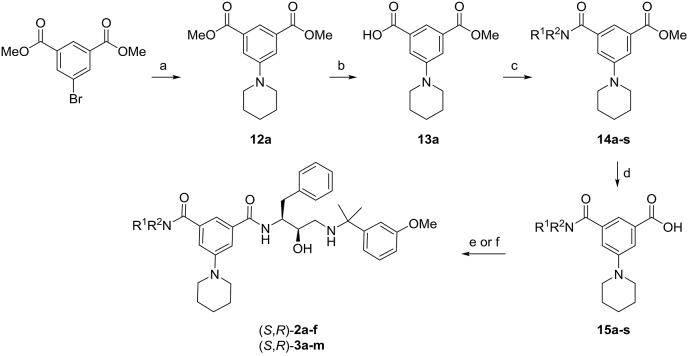
Scheme 2.
Synthesis of Plm inhibitors (S,R)–2a-f and (S,R)–3a-m. Reagents and conditions: a) piperidine (1 equiv), Pd(OAc)2 (5 mol%), rac-BINAP (5 mol%), Cs2CO3 (1.5 equiv), toluene, 100 °C, 18 h, 88%. b) General procedure A: aqueous 1 M NaOH (1 equiv), MeOH, rt, 16 h c) General procedure B: R1R2NH (1.2 equiv), HBTU (1 equiv), NEt3 (2 equiv), DMF, rt, 2 h d) General procedure C: aqueous 1 M NaOH (1.5 equiv), MeOH, 50 °C, 18 h e) General procedure D: amino alcohol (R,S)–11 (1.0 equiv), HBTU (1.0 equiv), NEt3 (4 equiv), DMF, rt, 16 h f) amino alcohol rac–11 (1.0 equiv), HBTU (1.0 equiv), NEt3 (4 equiv), DMF, rt, 16 h; then separation of enantiomers by HPLC on chiral stationary phase (Chiralpak-ID).