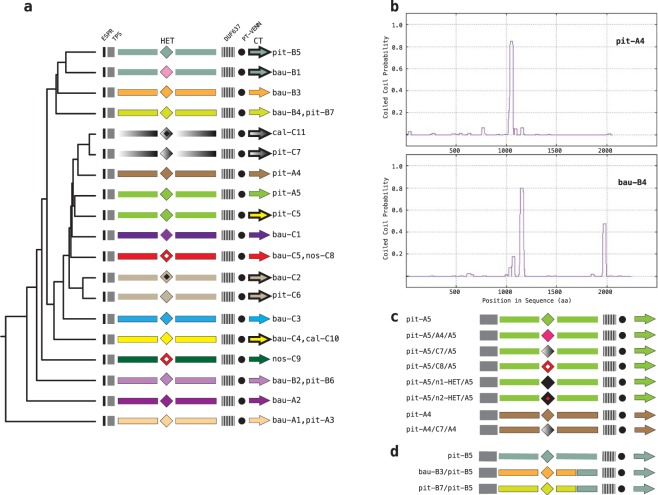
Figure 2.
Modular organization of type-I CdiA proteins. (a) The cladogram was generated from a ClustalW alignment of the reported proteins. ESPR (extended signal peptide region), TPS (two-partner secretion domain), HET (Heterogeneity region), DUF637 (domain of unknown function 637), PT-VENN (pretoxin PT-VENN domain) and CT (C-terminal toxic region) are shown. Homologous protein regions are coloured similarly. Similar CT regions are outlined (b) Coiled-coil conformation of pit-A4 and bau-B4 CdiA proteins predicted by the MARCOIL program. (c) Swapping of HET modules. Chimeric pit-A5 and pit-A4 proteins are shown. (d) Chimeric pit-B5 CdiA proteins. In panels a, c and d the proteins are not drawn to scale.