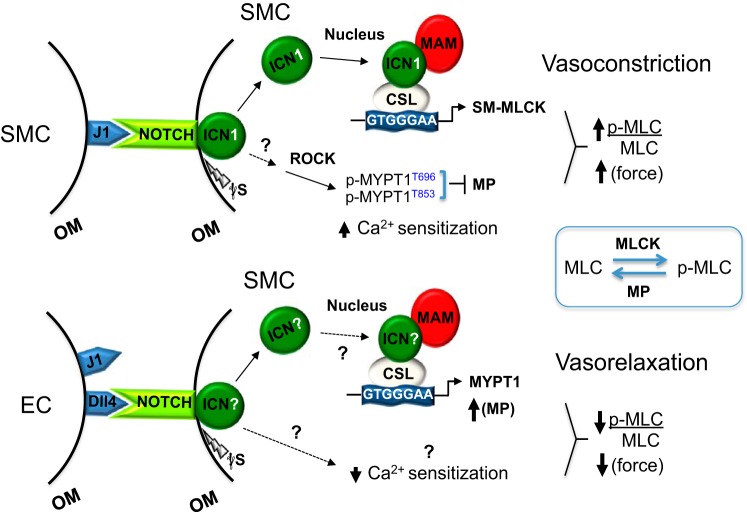
Fig. 9.
Model of Notch ligand-directed modulation of arterial vasoreactivity. Smooth muscle (SM) Jagged1 (J1) ligand engages Notch receptors on neighboring smooth muscle cells (SMCs) in the tunica media. These homotypic cell interactions trigger γ-secretase (γS) cleavage, release of intracellular Notch1 (ICN), and its translocation to the nucleus, where it targets and transcriptionally activates the myosin light chain kinase (MLCK) promoter. Jagged1 also stimulates Rho kinase (ROCK) phosphorylation of myosin phosphatase regulatory subunit 1 (MYPT1) residues Thr696 and Thr853 to inactivate myosin phosphatase (MP; i.e., Ca2+ sensitization). These combined effects result in a high phosphorylated myosin light chain (p-MLC)-to-total MLC ratio and force production. Though our prior report and data herein implicate the Notch1 receptor in transducing Jagged1-mediated vasoconstriction, it is possible that other Notch receptors may be used. Furthermore, how Notch signaling activates ROCK remains under investigation. Impairments in force production due to smooth muscle Jagged1 deficiency suggest that endothelial cell (EC) Jagged1 may be unable to confer comparable vasoconstrictor signals. In contrast to Jagged1, delta-like protein-4 (Dll4) is only expressed in the endothelium of arteries, which therefore suggests engagement of smooth muscle Notch receptors (as yet unidentified) through heterotypic cell interactions. Dll4 increases smooth muscle MYPT1 transcript and protein levels, suggesting that MYPT1 may be a target of Dll4-Notch activation. The heightened levels of MYPT1 support increased MP activity and vasorelaxation as reflected in relatively low p-MLC-to-total MLC ratios. The possibility that Dll4 alters Ca2+ sensitization remains to be determined. CSL, DNA-binding protein (CBF-1, suppressor of Hairless, Lag-2; also known as RBPJκ); MAM, Mastermind; OM, outer membrane; p-MYPT1, phosphorylated MYPT1 (residues Thr696 and Thr853). See text for further details.