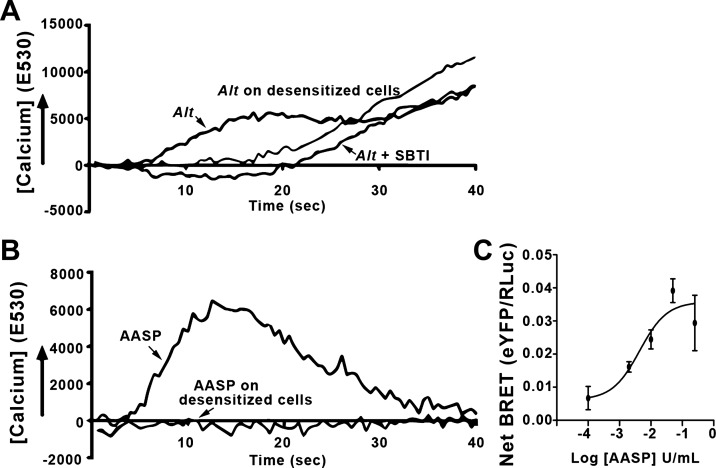
Fig. 10.
Alternaria (Alt) alkaline serine protease (AASP) induces protease-activated receptor-2 (PAR2)-dependent Ca2+ and β-arrestin signaling. A and B: Kirsten virus-transformed rat kidney cells (KNRK) cells transfected with PAR2 were loaded with the Ca2+ sensitive dye Fluo-4 and treated with Alt filtrate (A) or AASP from High-Q column fraction F4 (B) from Fig. 9B. Alt filtrate induced a protease-sensitive Ca2+ transient that required functional PAR2, as both soybean trypsin inhibitor (SBTI) and desensitization prevented the primary Ca2+ signaling. AASP induced a Ca2+ transient that required functional PAR2 as desensitization eliminated the Ca2+ signal. C: bioluminescence resonance energy transfer (BRET) was measured upon addition of increasing concentrations of AASP, as described in Fig. 1C. Half-maximal BRET value (BRET50) for AASP (2.0 U/ml) was similar to what was determined for trypsin (2.5 U/ml). AASP is sufficient to fully induce signaling pathways via PAR2; n ≥ 3 for all experiments (A and B) and experimental time points (C).