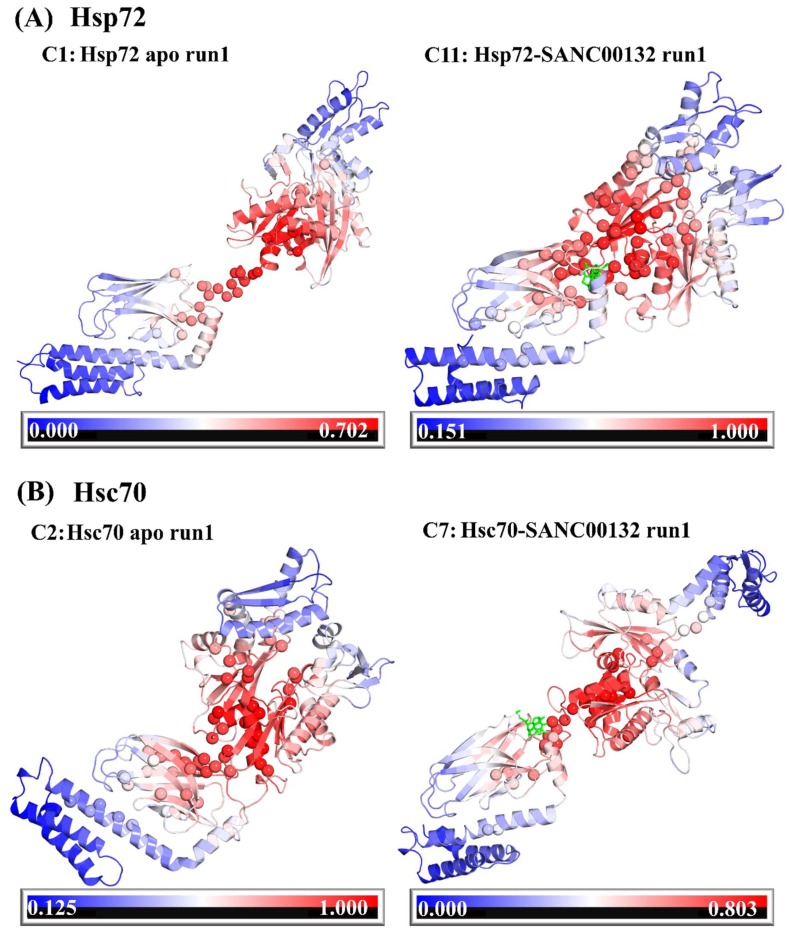
Figure 6.
Structural mapping of average L and average BC on SANC00132-free and SANC00132-bound Hsp72 and Hsc70 representative structures. Structures were retrieved from the largest clusters occupying important free-energy basins conformers (A: C1 (73 ns), C11 (82 ns), and B: C2 (56 ns), C7 (96 ns) in relation to Figure 3). Structures were coloured based on normalised average L values from red (lowest value; highly accessible) to blue (highest value; limited accessibility). Residues bearing the highest BC indices are indicated as spheres. First, both Hsp72 and Hsc70 images depict the global effects of ligand binding on structure compaction as described by Rg and interdomain distance results. Binding of SANC00132 to Hsp72 promotes intraprotein reorganisation from a loosely packed (more elongated) ligand-free 3D structure to a more compact complex. The opposite holds true for Hsc70. Second, it is evident that residue communities with significant average L and average BC values are populated at the interdomain linker, nucleotide, and substrate binding sites. SANC00132 is represented in sticks and coloured green.