Abstract
The extension of π-conjugation of polycyclic aromatic hydrocarbons (PAHs) via alkyne benzannulation reactions has become an increasingly utilized tool over the past few years. This short review will highlight recent work of alkyne benzannulations in the context of large nanographene as well as graphene nanoribbon synthesis along with a brief discussion of the interesting physical properties these molecules display.
Keywords: alkyne, benzannulation, nanographene, polycyclic aromatic hydrocarbon, graphene nanoribbon
1. Introduction
The term “nanographenes (NGs)” has recently become a popular term used to describe relatively large polycyclic aromatic hydrocarbons (PAHs) [1]. These molecules represent discrete sections of graphene, a material with its own interesting chemical and physical properties [2,3]. There has been increasing interest in the synthesis of NGs due to their use in applications such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and in solar-cell applications [4,5,6,7,8,9,10,11,12]. The attraction to NGs is that these “soft” materials have useful physical properties that can be easily tuned through structurally changes and/or substitution. A related class of compounds made of long, narrow strips or ribbons of graphene, known as “graphene nanoribbons (GNRs),” with large aspect ratios, have shown ideal semiconducting properties for potential use in OFETs [13,14,15]. The development of bottom-up synthetic tools for the production of GNRs has been an area of recent interest for the purpose of imparting solubility and tuning of the material properties. This short review will discuss the state of the art for NG and GNR synthesis with a focus on alkyne benzannulations [16] as the key chemical transformation.
2. Catalyst- and Reagent-Free Alkyne Benzannulations
2.1. Alkyne Benzannulations via Pyrolysis
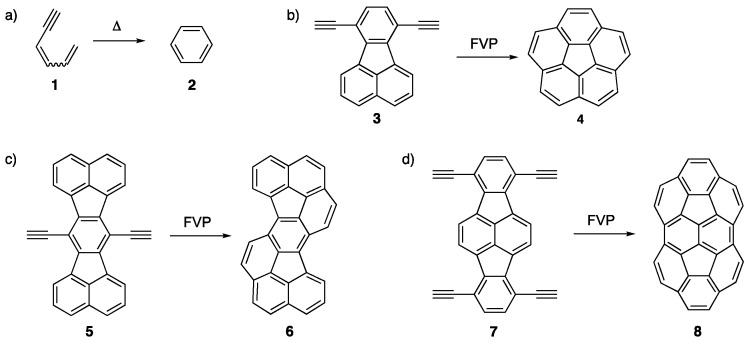
In 1969, Hopf and Musso showed that pyrolysis of cis-1,3-hexadien-5-yne 1 leads to the formation of benzene 2 (Scheme 1a) [17]. Pyrolysis of analogous molecules containing the 1,3-hexadien-5-yne substructure have led to small NGs such as naphthalenes [18,19]. Scott and coworkers utilized flash vacuum pyrolysis (FVP) for the conversion of 3 to corannulene 4, a bowl-shaped NG, which involves a two-fold alkyne benzannulation reaction (Scheme 1b) [20]. Shortly after this, others adopted this procedure to produce other non-planar NGs such as 6 and 8 (Scheme 1c,d) [21,22].
Scheme 1.
(a) Benzene 2 from the pyrolysis of 1,3-hexadien-5-yne 1 [17]. (b) FVP of 3 to produce corannulene 4 [20]. Two-fold [21] and four-fold alkyne benzannulations [22] to afford bowl-shaped NGs (c) 6 and (d) 8, respectively.
2.2. Alkyne Benzannulatinos via Photocyclizations
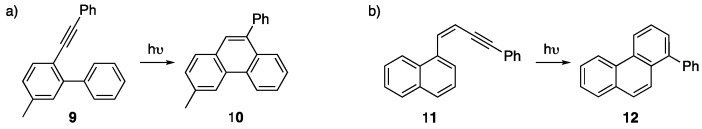
Photochemical alkyne benzannulations to afford large NGs are rare but there have been a few examples reported of smaller NGs being formed this way. For example, photocyclization of 2-ethynylbiphenyls such a 9 (Scheme 2a) [23] and 1,4-diaryl-1-buten-3-ynes 11 (Scheme 2b) [24,25] is known to afford phenanthrene products 10 and 12. With the success of photochemical alkyne benzannulations of smaller NG systems, the relatively underexplored area of larger NGs synthesized in this manner appears to be ripe with low-lying fruit.
Scheme 2.
Alkyne benzannulation of (a) 2-ethynylbiphenyl derivatives 9 [23] and (b) 1,4-diaryl-1-buten-3-ynes 11 [24,25] via a photocyclization reaction to afford phenanthrene products 10 and 12.
3. Alkyne Benzannulations Promoted by Electrophilic Reagents
Iodonium Salt- or Iodine Monochloride (ICl)-Induced Alkyne Benzannulations
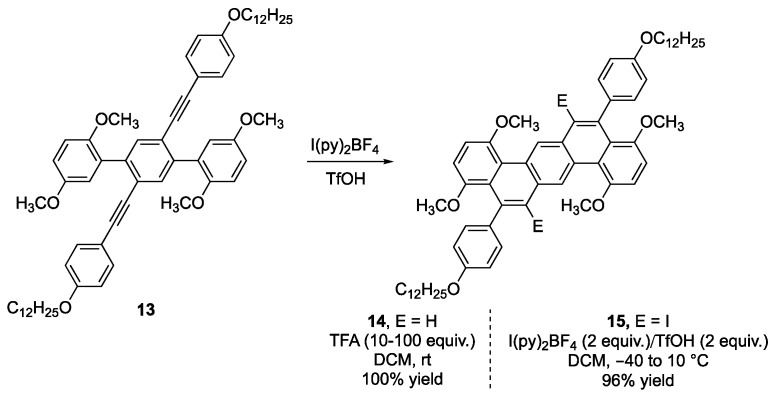
Electrophilic iodine reagents are an excellent way of cyclizing alkynes with neighboring aryl groups to produce a new benzene ring [26]. This methodology has the added advantage of providing a functional handle in the product for further chemical elaboration. This chemistry was first developed in 1988 by Barluenga and coworkers in which they used I(py)2BF4 as an electrophilic iodine source in the presence of TfOH to produce iodocyclohexene products from 1,4-diphenyl-1-butyne [27]. Swager and coworkers utilized this reagent system to cyclize terphenyl derivative 13 to give a mixture of halogenated and non-halogenated NG products 14 and 15 (Scheme 3) [28]. If the incorporation of an iodide group in the final product is desired, using equal equivalents of TfOH and I(py)2BF4 resulted in good yields of the halogenated product 15.
Scheme 3.
Electrophilic benzannulation with either an iodonium salt or a Brønsted acid [28].
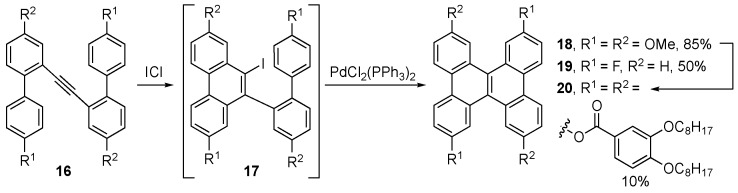
Liu and coworkers took advantage of an ICl-induced alkyne benzannulation of bis(biaryl)acetylenes 16 to arrive at iodide-functionalized phenanthrene intermediates 17 (Scheme 4) [29]. These intermediates are poised for a palladium-catalyzed intramolecular direct arylation to arrive at dibenzo[g,p]chrysenes in good to excellent overall yield. This route allowed for the synthesis of this class of NGs bearing both electron-rich (18) and electron-poor (19) substituents. This was important as Liu and coworkers were able to demonstrate that dibenzo[g,p]chrysenes functionalized with electron-withdrawing groups have larger HOMO/LUMO energy gaps (3.10–3.18 eV) and lower quantum yields (Φ = 12.4–16.8%) than derivatives with electron-donating groups (2.84–2.91 eV, Φ = 26.0–48.7%) [30]. They were also able to convert 18 to a liquid crystalline derivative 20 in 10% overall yield and this material displayed reasonable hole and electron transport in thin films. This work nicely demonstrates that physical properties of dibenzo[g,p]chrysene can be tuned through substituent effects.
Scheme 4.
ICl-induced benzannulation used in the synthesis towards dibenzo[g,p]chrysene derivatives [29].
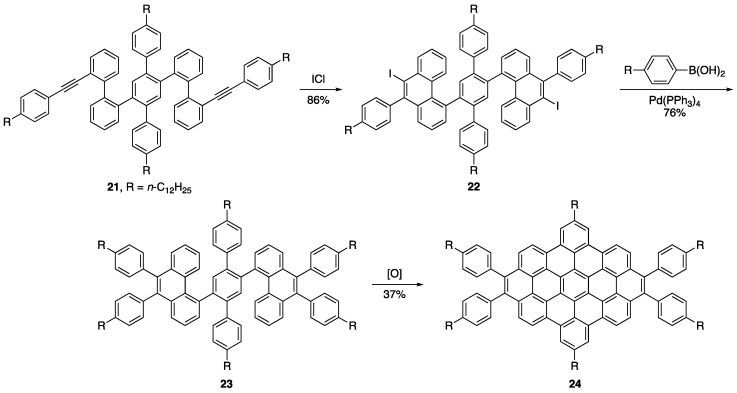
The Müllen group recently used an ICl-induced two-fold alkyne benzannulation of compound 21 to generate two new iodinated phenanthrene units in product 22 (Scheme 5) [31]. They demonstrated the crosscoupling utility of these intermediates by generating compound 23, which was further oxidized under Scholl conditions [32] to afford zigzag nanographenes 24. The formation of NG intermediates with useful coupling handles has also been used towards the formation of conjugated polymers [33].
Scheme 5.
Müllen and coworkers synthesis of zigzag NGs [31].
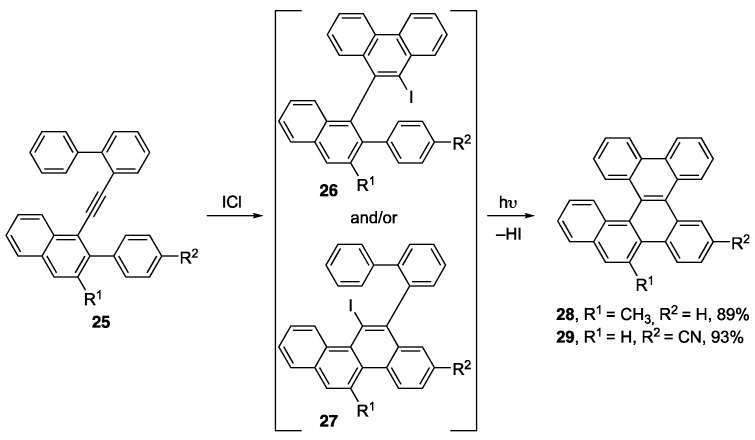
Molecular structure can greatly affect the properties exhibited by the bulk material, hence, it is important to understand how subtle changes to the molecular structure affect the HOMO/LUMO energy gaps, crystal packing, or UV-vis absorption and emission [34]. The way in which the twisted molecular structure of helicenes pack was of interest to the Alabugin group, who made [5]helicene-like compounds under metal-free conditions [35]. The reaction proceeds via an ICl-promoted alkyne benzannulation of 25 to afford either iodophenanthrene 26, iodochrysene 27, or a mixture of both (Scheme 6). This mixture was subjected to a cyclodehydroiodination reaction to afford [5]helicene derivatives 28 and 29, which were the two derivatives that afforded single crystals suitable for X-ray crystallography. It was found that the 3D crystal architecture significantly varied from the placement of the functional groups around the [5]helicene skeleton, but the calculated HOMO/LUMO energy gaps remained quite similar in value for most of the [5]helicenes synthesized in the study (2.51–2.68 eV).
Scheme 6.
Synthetic route to [5]helicene-like compounds [35].
Electrophilic iodine-mediated alkyne benzannulations have proven to be an excellent tool for the synthesis of functionalized nanographenes [29,31,36,37,38]. The products are often achieved in high yields and provide a useful handle for further chemical transformations.
4. Radical-Mediated Alkyne Benzannulations
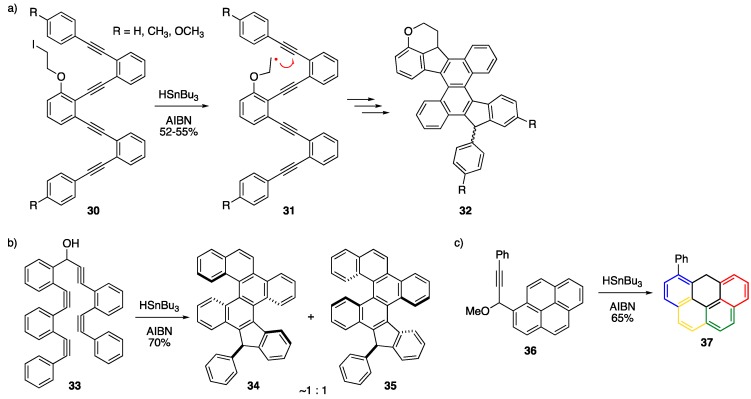
The Alabugin group has done significant work over the past few years using high-energy alkyne-containing substrates to produce a host of NGs through thermodynamically “downhill” alkyne benzannulations [35,39,40,41,42,43,44]. Their recent work stems from their initial discovery that tin-mediated radical cascade cyclizations of alkynes can be used to afford substituted benzo[a]indeno[2,1-c]fluorenes and other products [45]. Alabugin and Byers later used this strategy to cleverly stitch together larger NGs 32 (Scheme 7a) [40]. In this single reaction, the authors impressively generate five new rings and five new carbon-carbon bonds in excellent yield (ca. 93% yield per step), which demonstrates the exceptional chemoselectivity of radical formation and regioselectivity of the initial cyclization on the internal alkyne, as depicted in Scheme 7a. The Alabugin group later came up with a “traceless” version of this reaction to get a range of NG products including the conversion of 33 to arrive at a mixture of helical systems such as 34 and 35 (Scheme 7b) [42]. The chemistry leaves the product with a useful SnBu3 handle that their group demonstrated could be further reacted with electrophiles or used in Stille crosscoupling reactions. In 2018, and perhaps fortuitously timed with the 2018 Olympic Winter Games, the Alabugin group extended their methodology to peri-cyclizations, allowing them to synthesize a number of NGs including olympicene derivative 37 (Scheme 7c) [44].
Scheme 7.
Radical mediated cascade alkyne benzannulations to arrive at (a) NG 32 [40], (b) helical NGs 34/35 [42], and (c) olympicene 37 [44].
5. Acid-Mediated Alkyne Benzannulations
As demonstrated in Section 3, electrophilic reagents can be an effective way to promote 6-endo-dig cyclizations of alkynes to afford a new benzene ring and provide larger NG structures. Other electrophiles, such as acids, have also been effective in alkyne benzannulations over the past 30 years.
5.1. Brønsted Acid Catalyzed Alkyne Benzannulations
In Section 3 we highlighted how iodonium reagents can be used to promote alkyne benzannulations. In many cases, the proto (R = H, Scheme 3) product is desired and thus would require a second step of lithium-halogen exchange and protonation to arrive at the desired product [46]. Perhaps the simplest electrophile for effecting an alkyne benzannulations reaction to arrive directly at the proto product would be a proton. Some of the earliest work of alkyne benzannulations came from the Swager group and during an early study of electrophile-induced alkyne cyclizations using iodonium (Scheme 3) they noted that the use of TfOH or TFA alone very effectively promoted alkyne benzannulations to arrive at NG products [28]. This Brønsted acid-promoted benzannulation was hypothesized to occur through a 6-endo-dig pathway due to the carbocation stability through resonance with an electron-rich arene moiety attached to the acetylene [46]. Having an electron-rich aryl group proved to be essential for the success of this type of alkyne benzannulation in other examples of NG syntheses [47,48,49,50,51,52].
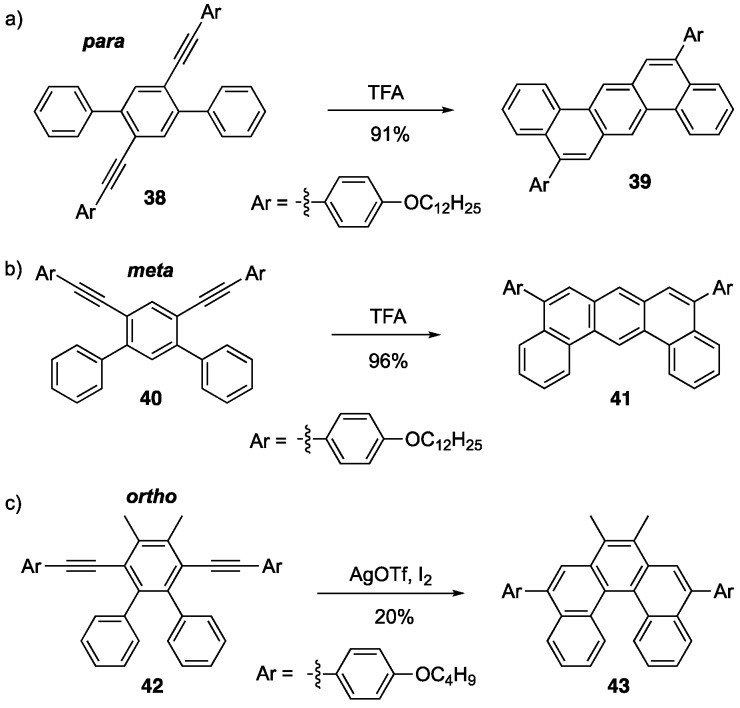
Swager and coworkers later explored their two-fold alkyne benzannulation reaction by looking at various substitution patterns of diethynylterphenyl systems in which the two aryl (Ar) groups participating in the alkyne benzannulation reaction were oriented para- (38), meta- (40) and ortho- (42) to each other to arrive at NGs 39, 41, and 43, respectively (Scheme 8) [46]. Attempting to cyclize systems with more steric strain, as the case with the ortho-terphenyl system 42, using Brønsted acids only provided trace amounts of desired products. The authors found that conducting the reaction in the presence of silver triflate and iodine resulted in the desired product in 20% yield. What was surprising is that there was no iodine detected in the NG product under these conditions.
Scheme 8.
Brønsted acid-induced alkyne benzannulations of (a) para- (38), (b) meta- (40) and (c) ortho-substituted (42) terphenyl systems to afford NGs [46].
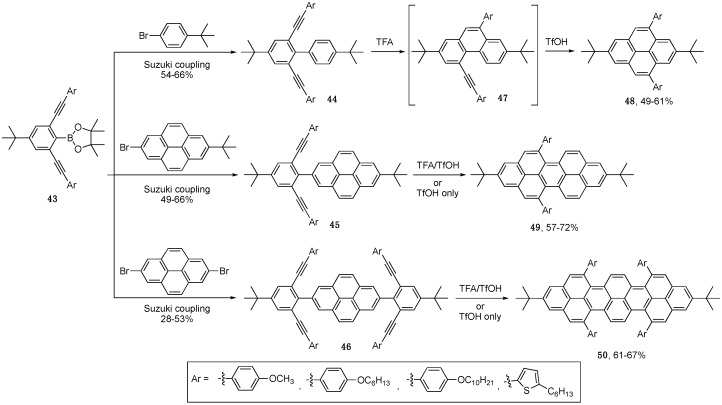
The Chalifoux group has recently adapted Swager’s alkyne benzannulation conditions to afford a host of nanographenes [47,48,49,50]. In one study, they are able to generate diynes 44 and 45 or tetrayne 46 intermediates via Suzuki crosscoupling of boronic ester 43 and various brominated aromatics (Scheme 9) [48]. The idea here was to invoke two benzannulation reactions on both ortho positions of the same aryl moiety, whereas in Swager’s examples they invoke only one alkyne benzannulation on one ortho position of the neighboring aryl group [28,46]. The authors thought this would allow them to rapidly access larger fused NG systems with greater lateral conjugation. However, they found that under Swager conditions (TFA), cyclization of compound 44 only produced monocyclized phenanthrene product 47, albeit quantitatively. The explanation for incomplete cyclization in this case, as compared to systems reported by Swager and coworkers [28,46], is that the first alkyne cyclization causes planarization to form rigid phenanthrene intermediate 47. This leads to poor orbital interaction between the second (remaining) alkyne and the remaining ortho position, resulting in a much higher barrier for the second benzannulation. Adding excess amounts of TFA along with refluxing the reaction mixture did lead to pyrene product 48, albeit in low yield. It was found that a stronger acid, such as TfOH, could be added to induce the second alkyne benzannulation to afford good yield of pyrene product 48. This protocol proved to be an effective method for synthesizing highly soluble peropyrene and teropyrene products 49 and 50, respectively. The two-step protocol of adding TFA followed by TfOH was useful for characterizing and studying the partially cyclized intermediates but the final nanographene products could be cleanly formed directly using just TfOH. The alkyne benzannulation reaction using Brønsted acid requires the presence of electron-rich ethynylaryl groups in the substrate and thus thiophene substituents are also tolerated [49]. The photophysical properties of these compounds were studied and, as expected, there is a red-shift in both the absorbance and emission in going from 48, to 49, to 50. Peropyrene products 49 display high extinction coefficients of 5.6 × 104 M−1·cm−1 at 467 nm and display green emission (λem = 486 nm) with quantum yields up to 64%. The teropyrenes 50 were significantly red-shifted with extinction coefficients of 2.8 × 105 m−1cm−1 at 572 nm and have red emission (λem = 590 nm) with quantum yields up to 61%.
Scheme 9.
Brønsted acid-promoted two-fold and four-fold alkyne benzannulations to afford pyrenes 48, peropyrenes 49, and teropyrenes 50 [48,49].
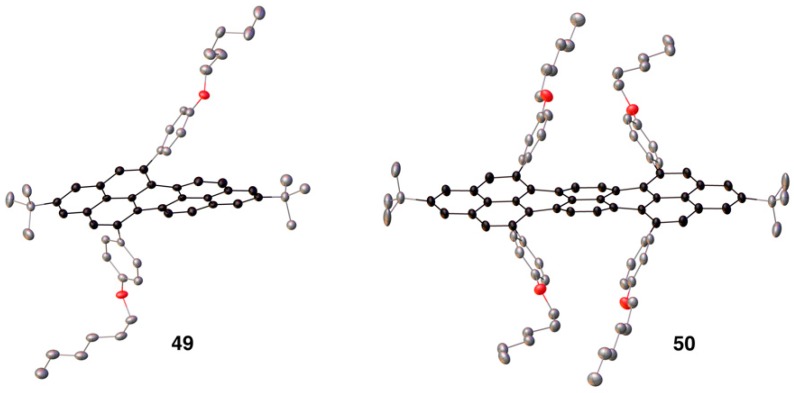
Single crystals suitable of X-ray crystallographic analysis were obtained for compounds 49 and 50 (Ar = p-hexyloxyphenyl) (Figure 1). As can be seen, the NGs adopt a non-planar structure due to steric hindrance within the newly formed bay regions. This feature, along with the ability to functionalize the NGs using this methodology, is likely responsible for the high solubility of these compounds. What is interesting is that the twist of peropyrene 49 causes the molecule to be chiral in the solid-state whereas teropyrene 50 has a pseudo plane of symmetry. In solution, it was hypothesized that the inversion barrier was low for 49 making it impossible to isolate each enantiomer under ambient conditions.
Figure 1.
X-ray crystal structures of peropyrene 49 and teropyrene 50.
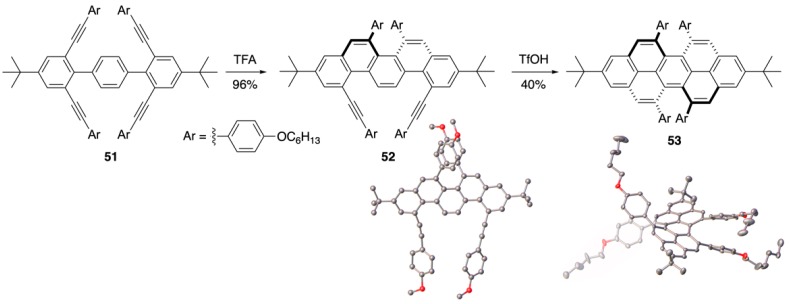
The Chalifoux group wondered if they could impart higher steric hinderance in the bay regions of a peropyrene through substitution in order to increase the inversion barrier and allow for isolation of the enantiomeric products. To probe the viability of synthesizing a persistently chiral peropyrene, the Chalifoux group synthesized tetrayne 51 and subjected it to their Brønsted acid-catalyzed alkyne benzannulation conditions (Scheme 10) [50]. Interestingly, using TFA alone resulted in a two-fold alkyne benzannulation in which the alkynes cyclized on the same side to produce chiral picene intermediate 52, which was unambiguously confirmed by X-ray crystallographic analysis (Ar = p-MeOC6H4). This cyclization mode was supported by calculations that determined a lower barrier of about 2 kcal/mol for this product. Treating 52 with TfOH resulted in complete cyclization to afford the first ever reported chiral peropyrene 53. The X-ray structure shows this molecule to be twisted from end-to-end by about 28°. The inversion barrier was determined to be ca. 29 kcal/mol making it possible to separate the enantiomers by chiral HPLC. An optical rotation [α] = value of + 1438 was determined for the pure enantiomer of (P,P)-53. Circular dichroism of 53 showed strong Cotton effects (Δε = ±100 M−1·cm−1 at 300 nm) and the compound also displayed circularly polarized luminescent properties, also observed in other helical NGs [53,54], making it potentially useful in chiroptic applications.
Scheme 10.
Four-fold alkyne benzannulation towards chiral peropyrenes 53 [50].
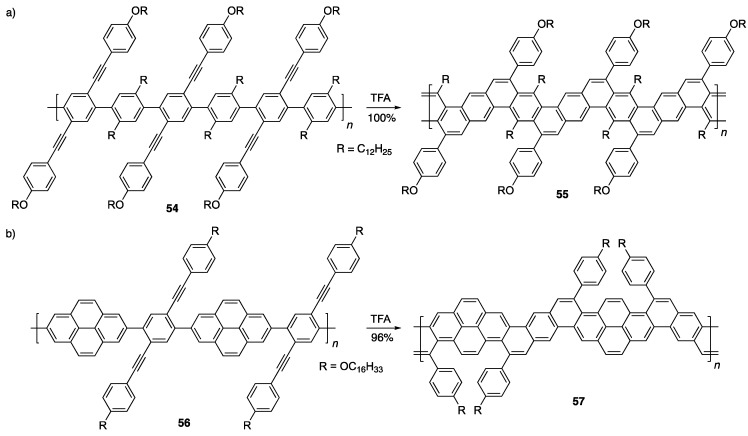
Bottom-up synthesis of graphene nanoribbons (GNRs) has been attempted long before the discovery of graphene itself and research towards the synthesis of GNRs is still being conducted today [14,55,56,57,58,59,60,61,62,63,64]. In 1970, Stille et al. reported the first bottom-up synthesis of a GNR, however, these carbon-based ladder polymers suffered from considerable insolubility [65]. It wasn’t until 1994 that the Swager group realized the potential of using a Brønsted acid-promoted alkyne benzannulation of polymer 54 as a means of creating functionalized, soluble GNRs 55 (Scheme 11a) [28]. Their strategy involved the use of thoughtfully designed monomers that included both electron-donating groups to ensure clean cyclization and alkyl substitution (R) along the backbone to ensure good regiocontrol. The polymers had high molecular weights of Mn = 45,000–55,000 g/mol and displayed an absorption edge at 468 nm. About two decades later, Wu, Zhao and coworkers used a similar strategy to synthesize more conjugated and rigid pyrene-based GNRs 57 (Scheme 11b) [66]. As expected, they observed roughly a 100 nm red-shift in the absorption edge to ca. 550 nm relative to GNR 55.
Scheme 11.
(a) Synthesis of GNRs 55 by Swager and coworker [28]. (b) Synthesis of expanded GNRs 57 by Wu, Zhao and coworkers [66].
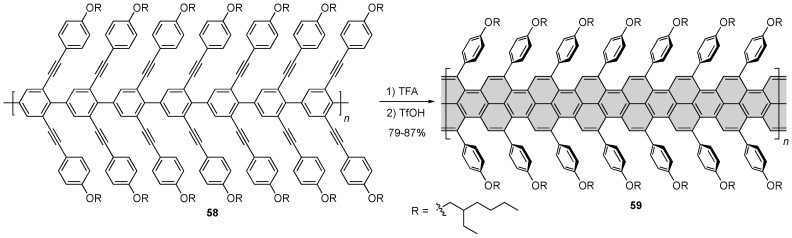
Taking a similar approach as with their nanographene synthesis (vide supra), Chalifoux and coworkers used a Brønsted acid-catalyzed alkyne benzannulation strategy to cyclize poly(2,6-dialkynyl-p-phenylene) 58 into a functionalized GNR 59 (Scheme 12) [67]. This flexible GNR was reported to have average lengths of 8 nm when the Suzuki polymerization was carried out in water and toluene as solvent and 35 nm when the Suzuki polymerization was carried out in water and THF as solvent. GNR 59 proved to be highly soluble in a number of common organic solvents allowing for its study by IR, Raman, NMR and UV/Vis/NIR spectroscopy. The GNR showed broad absorption in the visible and near-infrared spectrum with a large red-shift as compared to GNRs 55 and 57 (Scheme 11), displaying an absorption edge at 1200 nm, which corresponds to an optical bandgap of 1.03 eV.
Scheme 12.
Soluble GNR 59 reported by Chalifoux and coworkers [67].
The use of Brønsted acid-promoted alkyne benzannulations has proven to be a convenient route towards the synthesis of NGs and GNRs. However, the limitation that electron-rich ethynylaryl groups are needed for a successful 6-endo-dig cyclization under these conditions makes it a challenge to tune the electronic and optical properties through substituent effects.
5.2. π-Lewis Acid Catalyzed Alkyne Benzannulations
Over the last ca. 30 years, significant optimization of the alkyne benzannulation reaction has led to the discovery of a variety of reagents that promote cyclization in moderate to good yields under relatively mild reaction conditions. There have been numerous examples where π-Lewis acids have been used to activate an alkyne towards cyclization [68,69,70,71,72,73,74,75]. The η2-metal complex formed with the alkyne makes it susceptible to nucleophilic attack from the adjacent aromatic ring leading to a new C-C bond. In most cases, strongly deactivating groups, such as esters, attached to the alkyne were found to favor the 5-exo-dig pathway, whereas activating groups almost exclusivity formed the 6-endo-dig product [76]. With some understanding to mechanism of this reaction, researchers have begun taking advantage for the purpose of synthesizing large NG systems.
5.2.1. Transition Metal-Catalyzed Cyclizations
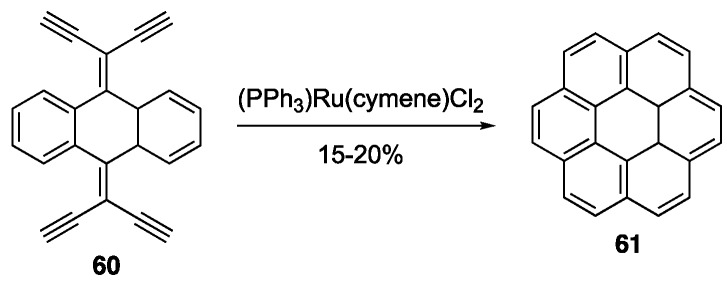
Ruthenium catalysts have been shown to be effective at effecting alkyne benzannulations to afford NGs [68,75,76,77,78,79,80,81]. NGs doped with boron and silicon have also been produced using Ru(II) catalysts [82]. Scott and coworker demonstrated that two- and even four-fold alkyne benzannulations reactions using (Ph3P)Ru(cymene)Cl2 could produce a host of NGs, including coronene 61 (Scheme 13) [77]. Liu and coworkers were able to improve the yield of coronene and extend the scope of NGs synthesized in this way using a more reactive Ru(II) catalyst system [78].
Scheme 13.
First synthesis of coronene 61 using a Ru(II)-catalyzed four-fold alkyne benzannulation [77].
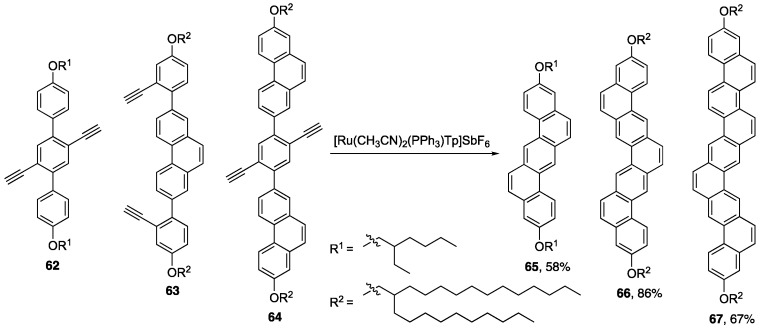
The Liu group was able to utilize Ru-catalysis to synthesize a series of NG ribbons of various lengths (Scheme 14) [79]. They later showed that substrates containing Ph2N groups were also tolerated in this reaction [80]. These second-generation ribbons were reported to have excellent quantum yields up to 96.3% with larger HOMO/LUMO energy gaps vs compounds 65–67.
Scheme 14.
Synthesis of GNRs 65–67 via a Ru(II)-catalyzed two-fold alkyne benzannulation by Liu and coworkers [79]. [Tp = tris(1-pyrazolyl)borate)]
Though Ru(II) catalysis is useful for the formation of NGs and GNRs, it is limited to cyclizing compounds that contain terminal acetylenes. As one might imagine, planarization of the system leads to decreased solubility and thus incorporation of appropriate solubilizing groups elsewhere in the substrate needs to be done if using this methodology.
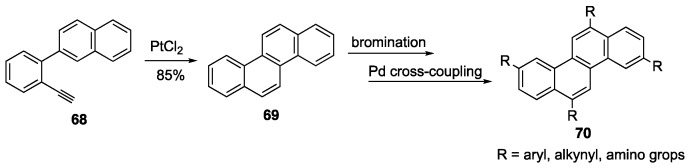
Alkyne benzannulations using Pt(II) catalysis has also become a popular method to arrive at various NGs [79,80,83,84,85]. For example, Liu and coworkers developed a four-step synthesis of chrysene 69 from cheap, commercially available starting materials with the key step involving a Pt(II)-catalyzed alkyne benzannulation of intermediate 68 (Scheme 15) [83]. Chrysene itself is a small NG that emits blue light at 383 nm with a quantum yield of 16% making derivatives of chrysene promising in OLED applications [86]. Studies on the effects that substituents have on the chrysene’s emission and quantum yield show that tetra- and disubstituted molecules still emit at blue wavelengths and the quantum yield can be increased to 49% [87]. Chrysene 69 can be further functionalized though bromination at the 3-, 6-, 9-, 12-postions to afford tetrabrominated chrysene which can undergo Pd-catalyzed crosscoupling to afford chrysene derivatives 70 [83,87]. An analogous version of this reaction has also been reported using microwave irradiation to arrive at other substitution patterns of chrysene [88].
Scheme 15.
Pt-catalyzed alkyne benzannulation to afford chrysene 69 [83,87].
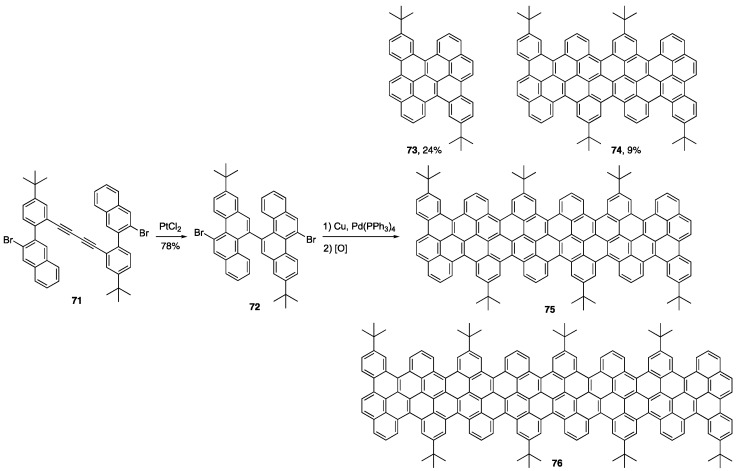
In an attempt to create cove-edged GNRs using solution-phase chemistry, Müllen and coworkers employed a Pt(II)-catalyzed alkyne benzannulation of 71 to synthesize chrysene-based dimer 72 (Scheme 16) [85]. Dimer 72 was subjected to Ullmann coupling followed by a Scholl oxidation to form a mixture of NGs 73–76. The authors noted that longer NGs 75 and 76 suffered from incomplete oxidative aryl-aryl coupling. X-ray crystallographic analysis of NG 74 showed that the carbon framework was nonplanar due to steric hindrance in the cove regions, adopting an “up-down” pattern that resulted in 74 being chiral in the solid-state. Not surprisingly, the optical bandgap in going from 73 to 74 shows a decrease as a function of length, with values 2.36 and 1.90 eV, respectively.
Scheme 16.
Cove-edge NGs produced from Pt(II)-catalyzed alkyne benzannulations [85].
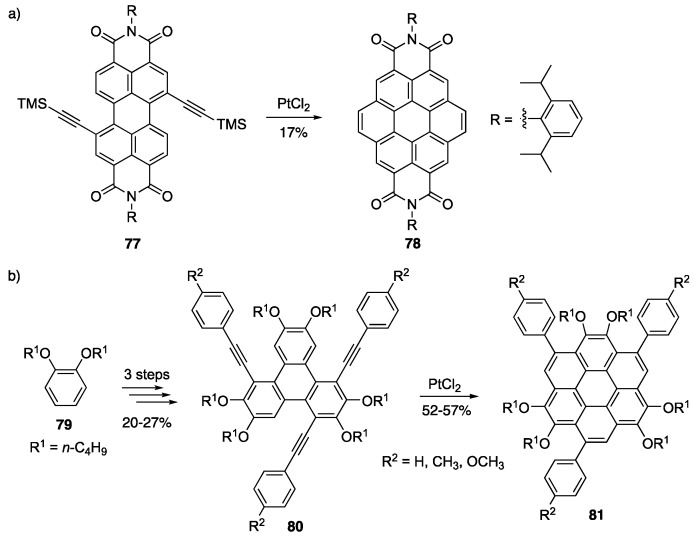
Due to their disk like shape and perfect π-conjugation on the outer most rings, coronene derivatives have become of great interest for applications such as discotic liquid crystals and organic electronics [89,90,91,92]. Some of the earliest synthetic routes to make coronenes have been riddled with long procedures and low yields [93,94]. Recently, an effort to optimize this synthesis has been made including the use of transition-metal assisted benzannulation [77,78]. Müllen and coworkers have recently used Pt(II)-catalyzed alkyne benzannulation of 77 with concomitant desilylation to produce coronene tetracarboxdiimide 78 (Scheme 17a) [95]. The synthetic approach towards functionalized coronenes was recently improved by Liu, Yin and coworkers by simplifying the starting material to a functionalized triphenylene moiety 79, which was converted to their key intermediate 80 in 3 steps (Scheme 17b) [96]. The key step was an alkyne benzannulation step using Pt(II)-catalysis to product functionalized coronenes 81. These functionalized coronene derivatives have good solubility in common organic solvents due to the presence of alkyl groups around the periphery of the molecule. They have optical energy gaps of 3.02–3.07 eV, showing no photodegradation when irradiated with white light (100 W) for 8 h and only minimal degradation after irradiation with UV light (6 W) after 8 h.
Scheme 17.
1Pt(II)-catalyzed benzannulation towards functionalized coronenes 79 [96].
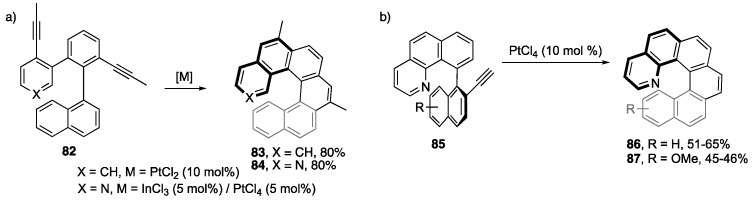
Various helicenes [97,98], and derivatives thereof, have been produced by Pt(II)-catalyzed alkyne benzannulations [99,100,101,102,103]. For example, the Storch group was able to synthesize [6]helicene 83 in only 6 steps using PtCl2 (Scheme 18a) [99]. Interestingly, the synthesis of aza[6]helicene 84 required a mixture of InCl3 and PtCl4 to effectively promote the alkyne benzannulation [100]. Wanting to extend the substrate scope of aza[6]helicenes, Fuchter and coworkers sought to make functionalized aza[6]helicene derivatives using the same benzannulation conditions as outlined by Storch [101]. They found that the reaction conditions, as reported, yielded very little product, even when up to half an equivalent of catalyst was used. Optimizing of the reaction conditions by changing solvents, increasing the temperature, and switching to PtCl4 gave better yields for the conversion of 85 to products 86 and 87 (Scheme 18b). The authors noted that the pre-cyclized atropisomers of 85 (R = H) could be separated by semipreparative chiral HPLC and cyclized to give enantioenriched (M)-86 (90% ee) and P-86 (92% ee).
Scheme 18.
(a) [6]Helicene 83 and aza[6]helicene 84 synthesis by Storch and coworkers [99]. (b) Aza[6]helicenes 86 and 87 synthesized by Fuchter and coworkers [101].
Surprisingly, the synthesis of large NGs using Au-catalysis is rare but has been successfully employed for alkyne benzannulations to afford various small NGs, including chiral helicene-like compounds [104], pyrenes [71,72,84], and phenanthrenes [69,75,76].
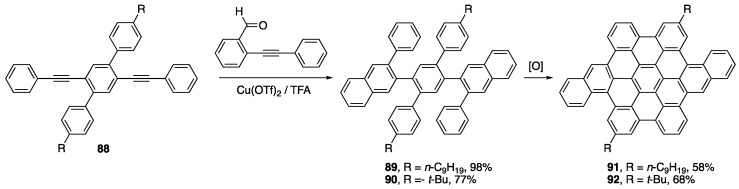
The Dichtel group has recently popularized the Asao-Yamamoto benzannulation [105]—a type of alkyne benzannulation catalyzed by Cu(II) and Brønsted acid that proceeds through a benzopyrylium intermediate—to afford a number of NG precursors that could be oxidized via the Scholl reaction to afford various NGs [106,107,108,109,110,111,112,113]. For example, treatment of diyne 88 under the reaction conditions resulted in a two-fold alkyne benzannulation to afford NG precursors 89 and 90 (Scheme 19) [111]. Compounds 89 and 90 were oxidized under Scholl conditions to afford NG derivatives 91 and 92. The Dichtel group has also utilized this strategy in a polymeric system to afford GNRs after Scholl oxidation [114].
Scheme 19.
Asao-Yamamoto alkyne benzannulation reported by Dichtel and coworkers towards NGs 91 and 92 [111].
5.2.2. Main Group π-Lewis Acid-Catalyzed Cyclizations
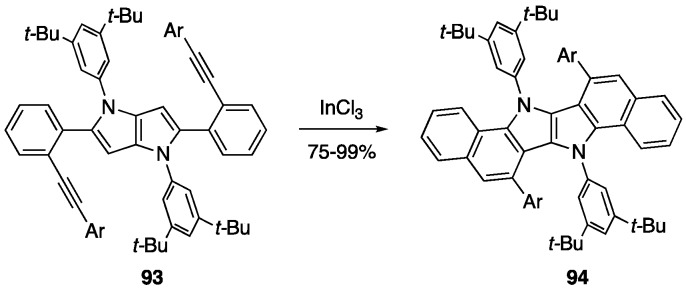
Other metal catalysts have successfully cyclized alkynes to afford a benzene ring, including GaCl3 [75,76], SbCl5 [115], and InCl3 [75,76]. In many cases, InCl3 has proven to be superior catalyst in terms of yield for 6-endo-dig cyclization of alkynes onto heteroaromatics and biphenyls containing haloalkyne substituents [76]. Gryko and coworkers took advantage of this by utilizing InCl3 to cyclize both electron-rich and electron-poor ethynylaryl groups onto pyrrolo[3,2-b]pyrroles 93 to afford π-expanded indolo[3,2-b]indole products 94 (Scheme 20) [70].
Scheme 20.
Synthesis of compounds 94 by a two-fold alkyne benzannulation using InCl3 [70].
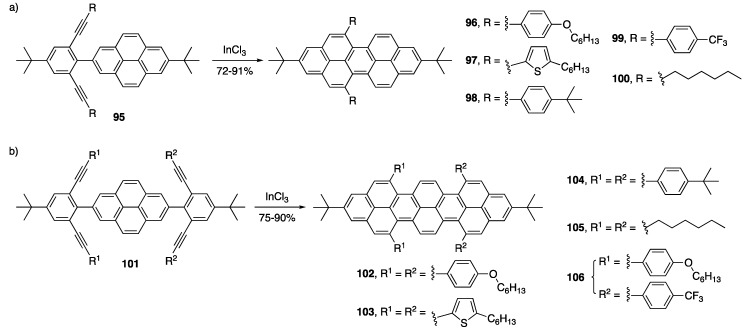
The Chalifoux group had shown that only electron-rich ethynylaryl substituents successfully cyclize using strong Brønsted acids to give NGs (vide supra). Turning to the work of Fürstner [75,76] and Gryko [70] as inspiration, the Chalifoux group explored the use of InCl3 in their two- and four-fold alkyne benzannulation towards the synthesis of peropyrenes and teropyrenes [116]. This catalyst system not only proved to be superior in terms of yield for the cyclization of electron-rich ethynylaryl substituents to produce peropyrenes 96 and 97 but allowed for the cyclization of electron-neutral 98, electron-poor 99, and even alkyl-substituted ethynylaryl groups 100 (Scheme 21a). This catalyst system was also able to promote a four-fold alkyne benzannulation to afford a broader scope of teropyrene products 102–105, including the differentially substituted “push-pull” derivative 106 (Scheme 21b). This broader scope of NGs allowed them to study the substituent effects on crystal packing, optical, and electrochemical properties.
Scheme 21.
InCl3-catalyzed alkyne benzannulation towards a broad scope of (a) peropyrenes 96–100 and (b) teropyrenes 102–106 [116].
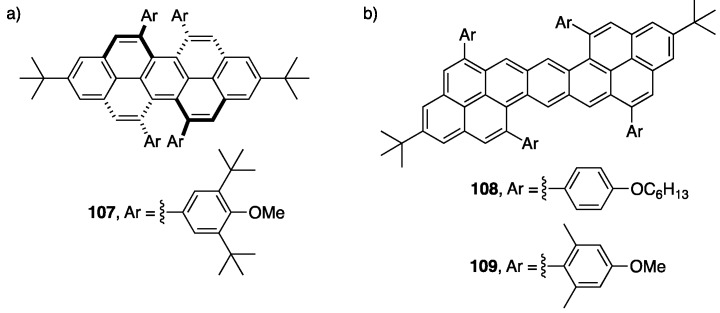
The use of indium(III) catalysts have not only opened the door to a broader scope of NG products available, but also for the cyclization of challenging substrates. Chalifoux and coworkers were able to use InCl3 to invoke a four-fold cyclization to afford highly contorted chiral peropyrenes, including the extremely sterically hindered derivative 107 (Figure 2a) [116]. This method proved to be more mild and efficient than what was reported using strong Brønsted acids such as TfOH [48]. The same group recently showed that other NG scaffolds are also accessible using indium(III) catalysis. A four-fold alkyne benzannulation was recently used in the synthesis of benzodipyrene (BDP) derivatives 108 and 109 (Figure 2b) [117]. This reaction required a stronger catalyst system that consisted of InCl3/AgNTf2 (1:1). The BDP molecules show close stacking of ca. 4 Å in the solid-state, as determined by X-ray crystallographic analysis. The BDPs also show excellent photostability and behave as 1O2 sensitizers with up to 70% quantum efficiency of 1O2 generation.
Figure 2.
(a) Sterically hindered chiral peropyrene 107 [116]. (b) BDPs 108 and 109 synthesized by a four-fold alkyne benzannulation [117].
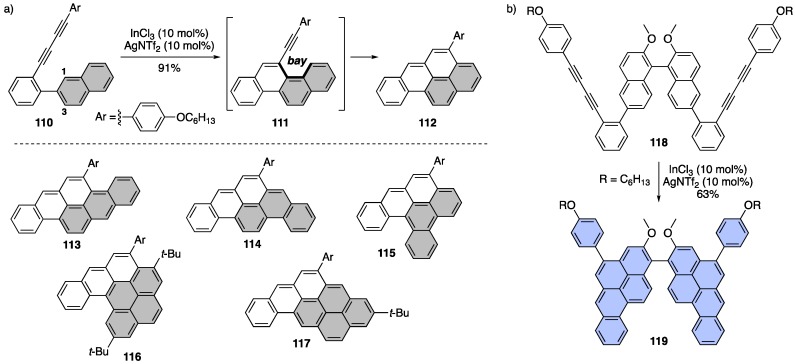
Recently, the very first domino alkyne benzannulation of diynes to make a host of irregular NGs was reported by the Chalifoux group [118]. This reaction was shown to be highly regioselective where diyne 110 cyclizes on carbon 1 of the naphthyl group rather than carbon 3, leading to intermediate 111 and a newly formed bay region (Scheme 22a). The new bay region in 111 is poised for a subsequent alkyne benzannulation to produce NG 112 in excellent yield. Regioselective cyclization onto various other PAH fragments (anthryl, phenanthryl, and pyrenyl) allowed the authors to demonstrate that this is a nice method for arriving at various NGs 113–117. The authors were also able to effect a four-fold cyclization of 118 to make four new carbon-carbon bonds and four new aryl rings in a single reaction to arrive at a large NG-based chiral “butterfly” molecule 119 that could serve as a new chiral ligand scaffold (Scheme 22b).
Scheme 22.
(a) Regioselective domino alkyne benzannulation of diynes to form various NGs 112–117. (b) Four-fold alkyne benzannulation towards a chiral butterfly ligand motif 119 [118].
6. Base-Mediated Alkyne Benzannulations
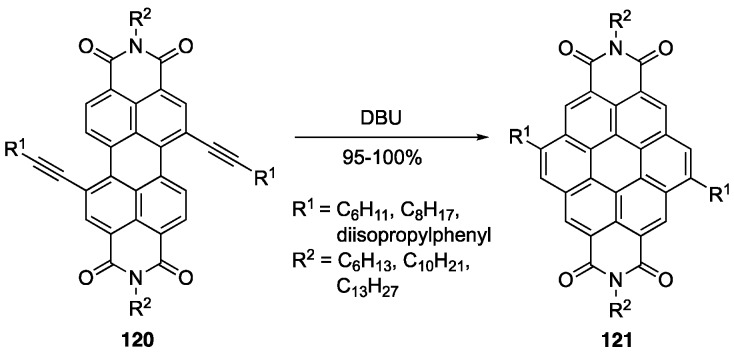
Coronene tetracarboxdiimides (CDIs) have attracted interest as solar collectors [119] and in lasers [120] due to their chemical, thermal, and photochemical stability. More recently, these derivatives have been of interest for their application in biological systems [121] as well as OLED and OFET materials [122]. Syntheses of these derivatives are lengthy and with poor yields. To optimize the synthetic routes to these CDIs, scientists have begun to use benzannulation methods to grow the number aromatic rings around the perylenediimide (PDI) core. Müllen and coworkers reported the synthesis of CDIs using a DBU-promoted alkyne benzannulation reaction of diethynyl-substituted PDIs 120 to arrive at alkylated CDI derivatives 121 in good yields (Scheme 23) [123]. Since this report, there have been many examples of base-induced alkyne benzannulations to produce various NGs [122,124,125], heteroatom-containing NGs [126], and polymers [127,128,129].
Scheme 23.
Base-mediated alkyne benzannulations towards CDIs 121 [123].
7. Conclusions
With the increasing interest in the use of alkyne benzannulation reactions to rapidly extend the π-conjugation towards NG structures, it will be interesting to see what new structures will be presented going into the future. The current toolbox has been expanded to include a number of electrophilic reagents, radical processes and catalysts, many of which offer advantages depending on what core structure is desired. This allows access to a broader scope of NGs, which is highly desirable as their photophysical, chemical, physical and electronic properties can be tuned through variation of their size and structure. With many of the methods discussed here being amenable to the incorporation of various functional groups into the NG and GNR precursors, chemistry now have a way to also “fine tune” the physical properties of these highly desirable materials.
Author Contributions
Writing—original draft preparation, A.D.S.; funding acquisition, writing—final draft preparation, review and editing, W.A.C.
Funding
This research was funded by the National Science Foundation via a CAREER award to WAC, grant number CHE-1555218.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Narita A., Wang X.-Y., Feng X., Müllen K. New Advances in Nanographene Chemistry. Chem. Soc. Rev. 2015;44:6616–6643. doi: 10.1039/C5CS00183H. [DOI] [PubMed] [Google Scholar]
- 2.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric Field Effect in Atomically Thin Carbon Films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Pérez L., Herranz M.a.Á., Martín N. The Chemistry of Pristine Graphene. Chem. Commun. 2013;49:3721–3735. doi: 10.1039/c3cc38950b. [DOI] [PubMed] [Google Scholar]
- 4.Wu J., Pisula W., Müllen K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007;107:718–747. doi: 10.1021/cr068010r. [DOI] [PubMed] [Google Scholar]
- 5.Sergeyev S., Pisula W., Geerts Y.H. Discotic Liquid Crystals: A New Generation of Organic Semiconductors. Chem. Soc. Rev. 2007;36:1902–1929. doi: 10.1039/b417320c. [DOI] [PubMed] [Google Scholar]
- 6.Laschat S., Baro A., Steinke N., Giesselmann F., Hägele C., Scalia G., Judele R., Kapatsina E., Sauer S., Schreivogel A., et al. Discotic Liquid Crystals: From Tailor-Made Synthesis to Plastic Electronics. Angew. Chem. Int. Ed. 2007;46:4832–4887. doi: 10.1002/anie.200604203. [DOI] [PubMed] [Google Scholar]
- 7.Pisula W., Feng X., Müllen K. Tuning the Columnar Organization of Discotic Polycyclic Aromatic Hydrocarbons. Adv. Mater. 2010;22:3634–3649. doi: 10.1002/adma.201000585. [DOI] [PubMed] [Google Scholar]
- 8.Mei J., Diao Y., Appleton A.L., Fang L., Bao Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013;135:6724–6746. doi: 10.1021/ja400881n. [DOI] [PubMed] [Google Scholar]
- 9.Ball M., Zhong Y., Wu Y., Schenck C., Ng F., Steigerwald M., Xiao S., Nuckolls C. Contorted Polycyclic Aromatics. Acc. Chem. Res. 2015;48:267–276. doi: 10.1021/ar500355d. [DOI] [PubMed] [Google Scholar]
- 10.Wöhrle T., Wurzbach I., Kirres J., Kostidou A., Kapernaum N., Litterscheidt J., Haenle J.C., Staffeld P., Baro A., Giesselmann F., et al. Discotic Liquid Crystals. Chem. Rev. 2016;116:1139–1241. doi: 10.1021/acs.chemrev.5b00190. [DOI] [PubMed] [Google Scholar]
- 11.Markiewicz J.T., Wudl F. Perylene, Oligorylenes, and Aza-Analogs. ACS Appl. Mater. Interfaces. 2015;7:28063–28085. doi: 10.1021/acsami.5b02243. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Kim B., Lee J., Shin H., Park Y.-I., Park J. Design of Fluorescent Blue Light-Emitting Materials Based on Analyses of Chemical Structures and their Effects. Mater. Sci. Eng. R-Rep. 2016;99:1–22. doi: 10.1016/j.mser.2015.11.001. [DOI] [Google Scholar]
- 13.Bai J., Huang Y. Fabrication and Electrical Properties of Graphene Nanoribbons. Mater. Sci. Eng. R-Rep. 2010;70:341–353. doi: 10.1016/j.mser.2010.06.019. [DOI] [Google Scholar]
- 14.Bennett P.B., Pedramrazi Z., Madani A., Chen Y.-C., Oteyza D.G.d., Chen C., Fischer F.R., Crommie M.F., Bokor J. Bottom-up Graphene Nanoribbon Field-Effect Transistors. Appl. Phys. Lett. 2013;103:253114. doi: 10.1063/1.4855116. [DOI] [Google Scholar]
- 15.Li X., Wang X., Zhang L., Lee S., Dai H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science. 2008;319:1229. doi: 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- 16.Hitt D.M., O’Connor J.M. Acceleration of Conjugated Dienyne Cycloaromatization. Chem. Rev. 2011;111:7904–7922. doi: 10.1021/cr2001542. [DOI] [PubMed] [Google Scholar]
- 17.Hopf H., Musso H. Preparation of Benzene by Pyrolysis of cis- and trans-1,3-Hexadien-5-yne. Angew. Chem. Int. Ed. 1969;8:680. doi: 10.1002/anie.196906801. [DOI] [Google Scholar]
- 18.Hofmann J., Schulz K., Altmann A., Findeisen M., Zimmermann G. Addition and Cyclization Reactions in the Thermal Conversion of Hydrocarbons with an Enyne Structure, 5. High-Temperature Ring Closures of 1,3-Hexadien-5-ynes to Naphthalenes—Competing Reactions via Isoaromatics, Alkenylidene Carbenes, and Vinyl-type Radicals. Liebigs Annalen. 1997;1997:2541–2548. doi: 10.1002/jlac.199719971218. [DOI] [Google Scholar]
- 19.Schulz K., Hofmann J., Findeisen M., Zimmermann G. Naphthalene Isotopomers from Isotope-Labelled Phenyl-Annelated 1,3-Hexadien-5-ynes Facilitate an Evaluation of Competing Radical Cycloisomerization Pathways. Eur. J. Org. Chem. 1998;1998:2135–2142. doi: 10.1002/(SICI)1099-0690(199810)1998:10<2135::AID-EJOC2135>3.0.CO;2-3. [DOI] [Google Scholar]
- 20.Scott L.T., Hashemi M.M., Meyer D.T., Warren H.B. Corannulene. A convenient new synthesis. J. Am. Chem. Soc. 1991;113:7082–7084. doi: 10.1021/ja00018a082. [DOI] [Google Scholar]
- 21.Masanori M., Hiroshi M., Masaaki S., Susumu O., Koji Y. Synthesis and Characterization of Hepta[5][5]circulene as a Subunit of C70 Fullerene. Chem. Lett. 1996;25:157–158. doi: 10.1246/cl.1996.157. [DOI] [Google Scholar]
- 22.Rabideau P.W., Abdourazak A.H., Folsom H.E., Marcinow Z., Sygula A., Sygula R. Buckybowls: Synthesis and ab Initio Calculated Structure of the First Semibuckminsterfullerene. J. Am. Chem. Soc. 1994;116:7891–7892. doi: 10.1021/ja00096a054. [DOI] [Google Scholar]
- 23.Lewis F.D., Karagiannis P.C., Sajimon M.C., Lovejoy K.S., Zuo X., Rubin M., Gevorgyan V. Solvent dependent photocyclization and photophysics of some 2-ethynylbiphenyls. Photochem. Photobiol. Sci. 2006;5:369–375. doi: 10.1039/B601751G. [DOI] [PubMed] [Google Scholar]
- 24.Tinnemans A.H.A., Laarhoven W.H. Photocyclisations of 1,4-Diarylbut-1-en-3-ynes. Part III. Scope and Limitations of the Reaction. J. Chem. Soc. Perkin Trans. 2. 1976:1115–1120. doi: 10.1039/p29760001115. [DOI] [Google Scholar]
- 25.van Arendonk R.J.F.M., de Violet P.H.F., Laarhoven W.H. The Mechanism of the Photocyclization of 1,4-Diarylbutenynes into Aryl-Substituted Aromatics. The Influence of Amines and Oxygen. Recl. Trav. Chim. Pays-Bas. 1981;100:256–262. doi: 10.1002/recl.19811000703. [DOI] [Google Scholar]
- 26.Yao T., Campo M.A., Larock R.C. Synthesis of Polycyclic Aromatics and Heteroaromatics via Electrophilic Cyclization. J. Org. Chem. 2005;70:3511–3517. doi: 10.1021/jo050104y. [DOI] [PubMed] [Google Scholar]
- 27.Barluenga J., González J.M., Campos P.J., Asensio G. Iodine-Induced Stereoselective Carbocyclizations: A New Method for the Synthesis of Cyclohexane and Cyclohexene Derivatives. Angew. Chem. Int. Ed. 1988;27:1546–1547. doi: 10.1002/anie.198815461. [DOI] [Google Scholar]
- 28.Goldfinger M.B., Swager T.M. Fused Polycyclic Aromatics via Electrophile-Induced Cyclization Reactions: Application to the Synthesis of Graphite Ribbons. J. Am. Chem. Soc. 1994;116:7895–7896. doi: 10.1021/ja00096a056. [DOI] [Google Scholar]
- 29.Li C.-W., Wang C.-I., Liao H.-Y., Chaudhuri R., Liu R.-S. Synthesis of Dibenzo[g,p]chrysenes from Bis(biaryl)acetylenes via Sequential ICl-Induced Cyclization and Mizoroki-Heck Coupling. J. Org. Chem. 2007;72:9203–9207. doi: 10.1021/jo701504m. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri R., Hsu M.-Y., Li C.-W., Wang C.-I., Chen C.-J., Lai C.K., Chen L.-Y., Liu S.-H., Wu C.-C., Liu R.-S. Functionalized Dibenzo[g,p]chrysenes: Variable Photophysical and Electronic Properties and Liquid-Crystal Chemistry. Org. Lett. 2008;10:3053–3056. doi: 10.1021/ol801029x. [DOI] [PubMed] [Google Scholar]
- 31.Feng X., Pisula W., Müllen K. From Helical to Staggered Stacking of Zigzag Nanographenes. J. Am. Chem. Soc. 2007;129:14116–14117. doi: 10.1021/ja075260w. [DOI] [PubMed] [Google Scholar]
- 32.Grzybowski M., Skonieczny K., Butenschön H., Gryko D.T. Comparison of Oxidative Aromatic Coupling and the Scholl Reaction. Angew. Chem. Int. Ed. 2013;52:9900–9930. doi: 10.1002/anie.201210238. [DOI] [PubMed] [Google Scholar]
- 33.Chan J.M.W., Kooi S.E., Swager T.M. Synthesis of Stair-Stepped Polymers Containing Dibenz[a,h]anthracene Subunits. Macromolecules. 2010;43:2789–2793. doi: 10.1021/ma902486y. [DOI] [Google Scholar]
- 34.Figueira-Duarte T.M., Müllen K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011;111:7260–7314. doi: 10.1021/cr100428a. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed R.K., Mondal S., Guerrera J.V., Eaton T.M., Albrecht-Schmitt T.E., Shatruk M., Alabugin I.V. Alkynes as Linchpins for the Additive Annulation of Biphenyls: Convergent Construction of Functionalized Fused Helicenes. Angew. Chem. Int. Ed. 2016;55:12054–12058. doi: 10.1002/anie.201606330. [DOI] [PubMed] [Google Scholar]
- 36.Chen T.-A., Liu R.-S. Synthesis of Polyaromatic Hydrocarbons from Bis(biaryl)diynes: Large PAHs with Low Clar Sextets. Chem. Eur. J. 2011;17:8023–8027. doi: 10.1002/chem.201101057. [DOI] [PubMed] [Google Scholar]
- 37.Chen T.-A., Liu R.-S. Synthesis of Large Polycyclic Aromatic Hydrocarbons from Bis(biaryl)acetylenes: Large Planar PAHs with Low π-Sextets. Org. Lett. 2011;13:4644–4647. doi: 10.1021/ol201854g. [DOI] [PubMed] [Google Scholar]
- 38.Yan Q., Cai K., Zhang C., Zhao D. Coronenediimides Synthesized via ICl-Induced Cyclization of Diethynyl Perylenediimides. Org. Lett. 2012;14:4654–4657. doi: 10.1021/ol301216p. [DOI] [PubMed] [Google Scholar]
- 39.Alabugin I.V., Gonzalez-Rodriguez E. Alkyne Origami: Folding Oligoalkynes into Polyaromatics. Acc. Chem. Res. 2018;51:1206–1219. doi: 10.1021/acs.accounts.8b00026. [DOI] [PubMed] [Google Scholar]
- 40.Byers P.M., Alabugin I.V. Polyaromatic Ribbons from Oligo-Alkynes via Selective Radical Cascade: Stitching Aromatic Rings with Polyacetylene Bridges. J. Am. Chem. Soc. 2012;134:9609–9614. doi: 10.1021/ja3023626. [DOI] [PubMed] [Google Scholar]
- 41.Pati K., dos Passos Gomes G., Alabugin I.V. Combining Traceless Directing Groups with Hybridization Control of Radical Reactivity: From Skipped Enynes to Defect-Free Hexagonal Frameworks. Angew. Chem. Int. Ed. 2016;55:11633–11637. doi: 10.1002/anie.201605799. [DOI] [PubMed] [Google Scholar]
- 42.Pati K., dos Passos Gomes G., Harris T., Hughes A., Phan H., Banerjee T., Hanson K., Alabugin I.V. Traceless Directing Groups in Radical Cascades: From Oligoalkynes to Fused Helicenes without Tethered Initiators. J. Am. Chem. Soc. 2015;137:1165–1180. doi: 10.1021/ja510563d. [DOI] [PubMed] [Google Scholar]
- 43.Pati K., Michas C., Allenger D., Piskun I., Coutros P.S., Gomes G.d.P., Alabugin I.V. Synthesis of Functionalized Phenanthrenes via Regioselective Oxidative Radical Cyclization. J. Org. Chem. 2015;80:11706–11717. doi: 10.1021/acs.joc.5b01014. [DOI] [PubMed] [Google Scholar]
- 44.Tsvetkov N.P., Gonzalez-Rodriguez E., Hughes A., dos Passos Gomes G., White F.D., Kuriakose F., Alabugin I.V. Radical Alkyne peri-Annulation Reactions for the Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene. Angew. Chem. Int. Ed. 2018;57:3651–3655. doi: 10.1002/anie.201712783. [DOI] [PubMed] [Google Scholar]
- 45.Alabugin I.V., Gilmore K., Patil S., Manoharan M., Kovalenko S.V., Clark R.J., Ghiviriga I. Radical Cascade Transformations of Tris(o-aryleneethynylenes) into Substituted Benzo[a]indeno[2,1-c]fluorenes. J. Am. Chem. Soc. 2008;130:11535–11545. doi: 10.1021/ja8038213. [DOI] [PubMed] [Google Scholar]
- 46.Goldfinger M.B., Crawford K.B., Swager T.M. Directed Electrophilic Cyclizations: Efficient Methodology for the Synthesis of Fused Polycyclic Aromatics. J. Am. Chem. Soc. 1997;119:4578–4593. doi: 10.1021/ja9642673. [DOI] [Google Scholar]
- 47.Yang W., Chalifoux W.A. Rapid π-Extension of Aromatics via Alkyne Benzannulations. Synlett. 2017;28:625–632. doi: 10.1055/s-0036-1588688. [DOI] [Google Scholar]
- 48.Yang W., Monteiro J.H.S.K., de Bettencourt-Dias A., Catalano V.J., Chalifoux W.A. Pyrenes, Peropyrenes, and Teropyrenes: Synthesis, Structures, and Photophysical Properties. Angew. Chem. Int. Ed. 2016;55:10427–10430. doi: 10.1002/anie.201604741. [DOI] [PubMed] [Google Scholar]
- 49.Yang W., Monteiro J.H.S.K., de Bettencourt-Dias A., Chalifoux W.A. New Thiophene-functionalized Pyrene, Peropyrene, and Teropyrene via a Two- or Four-fold Alkyne Annulation and their Photophysical Properties. Can. J. Chem. 2016;95:341–345. doi: 10.1139/cjc-2016-0466. [DOI] [Google Scholar]
- 50.Yang W., Longhi G., Abbate S., Lucotti A., Tommasini M., Villani C., Catalano V.J., Lykhin A.O., Varganov S.A., Chalifoux W.A. Chiral Peropyrene: Synthesis, Structure, and Properties. J. Am. Chem. Soc. 2017;139:13102–13109. doi: 10.1021/jacs.7b06848. [DOI] [PubMed] [Google Scholar]
- 51.Tovar J.D., Swager T.M. Exploiting the Versatility of Organometallic Cross-coupling Reactions for Entry into Extended Aromatic Systems. J. Organomet. Chem. 2002;653:215–222. doi: 10.1016/S0022-328X(02)01166-X. [DOI] [Google Scholar]
- 52.Mukherjee A., Pati K., Liu R.-S. A Convenient Synthesis of Tetrabenzo[de,hi,mn,qr]naphthacene from Readily Available 1,2-Di(phenanthren-4-yl)ethyne. J. Org. Chem. 2009;74:6311–6314. doi: 10.1021/jo901177e. [DOI] [PubMed] [Google Scholar]
- 53.Cruz C.M., Márquez I.R., Mariz I.F.A., Blanco V., Sánchez-Sánchez C., Sobrado J.M., Martín-Gago J.A., Cuerva J.M., Maçôas E., Campaña A.G. Enantiopure Distorted Ribbon-shaped Nanographene Combining Two-photon Absorption-based Upconversion and Circularly Polarized Luminescence. Chem. Sci. 2018;9:3917–3924. doi: 10.1039/C8SC00427G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruz C.M., Castro-Fernández S., Maçôas E., Cuerva J.M., Campaña A.G. Undecabenzo[7]superhelicene: A Helical Nanographene Ribbon as a Circularly Polarized Luminescence Emitter. Angew. Chem. Int. Ed. 2018;57:14782–14786. doi: 10.1002/anie.201808178. [DOI] [PubMed] [Google Scholar]
- 55.Narita A., Feng X., Müllen K. Bottom-Up Synthesis of Chemically Precise Graphene Nanoribbons. Chem. Rec. 2015;15:295–309. doi: 10.1002/tcr.201402082. [DOI] [PubMed] [Google Scholar]
- 56.Cai J., Ruffieux P., Jaafar R., Bieri M., Braun T., Blankenburg S., Muoth M., Seitsonen A.P., Saleh M., Feng X., et al. Atomically Precise Bottom-up Fabrication of Graphene Nanoribbons. Nature. 2010;466:470–473. doi: 10.1038/nature09211. [DOI] [PubMed] [Google Scholar]
- 57.Ruffieux P., Wang S., Yang B., Sánchez-Sánchez C., Liu J., Dienel T., Talirz L., Shinde P., Pignedoli C.A., Passerone D., et al. On-surface Synthesis of Graphene Nanoribbons with Zigzag Edge Topology. Nature. 2016;531:489–492. doi: 10.1038/nature17151. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y.-C., de Oteyza D.G., Pedramrazi Z., Chen C., Fischer F.R., Crommie M.F. Tuning the Band Gap of Graphene Nanoribbons Synthesized from Molecular Precursors. ACS Nano. 2013;7:6123–6128. doi: 10.1021/nn401948e. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo D.J., Veber G., Cao T., Bronner C., Chen T., Zhao F., Rodriguez H., Louie S.G., Crommie M.F., Fischer F.R. Topological Band Engineering of Graphene Nanoribbons. Nature. 2018;560:204–208. doi: 10.1038/s41586-018-0376-8. [DOI] [PubMed] [Google Scholar]
- 60.Vo T.H., Shekhirev M., Kunkel D.A., Morton M.D., Berglund E., Kong L., Wilson P.M., Dowben P.A., Enders A., Sinitskii A. Large-scale Solution Synthesis of Narrow Graphene Nanoribbons. Nat. Commun. 2014;5:3189. doi: 10.1038/ncomms4189. [DOI] [PubMed] [Google Scholar]
- 61.Vo T.H., Shekhirev M., Kunkel D.A., Orange F., Guinel M.J.F., Enders A., Sinitskii A. Bottom-up Solution Synthesis of Narrow Nitrogen-doped Graphene Nanoribbons. Chem. Commun. 2014;50:4172–4174. doi: 10.1039/C4CC00885E. [DOI] [PubMed] [Google Scholar]
- 62.Vo T.H., Shekhirev M., Lipatov A., Korlacki R.A., Sinitskii A. Bulk Properties of Solution-synthesized Chevron-like Graphene Nanoribbons. Faraday Discuss. 2014;173:105–113. doi: 10.1039/C4FD00131A. [DOI] [PubMed] [Google Scholar]
- 63.Daigle M., Miao D., Lucotti A., Tommasini M., Morin J.-F. Helically Coiled Graphene Nanoribbons. Angew. Chem. Int. Ed. 2017;56:6213–6217. doi: 10.1002/anie.201611834. [DOI] [PubMed] [Google Scholar]
- 64.Chalifoux W.A. The Synthesis of Non-planar, Helically Coiled Graphene Nanoribbons. Angew. Chem. Int. Ed. 2017;56:8048–8050. doi: 10.1002/anie.201702687. [DOI] [PubMed] [Google Scholar]
- 65.Stille J.K., Noren G.K., Green L. Hydrocarbon Ladder Aromatics from a Diels-Alder Reaction. J. Polym. Sci. A-Polym. Chem. 1970;8:2245–2254. doi: 10.1002/pol.1970.150080830. [DOI] [Google Scholar]
- 66.Zhu X., Wu Y., Zhou L., Wang Y., Zhao H., Gao B., Ba X. Synthesis of Pyrene-based Planar Conjugated Polymers and the Regioisomers by Intramolecular Cyclization. Chin. J. Chem. 2015;33:431–440. doi: 10.1002/cjoc.201400793. [DOI] [Google Scholar]
- 67.Yang W., Lucotti A., Tommasini M., Chalifoux W.A. Bottom-Up Synthesis of Soluble and Narrow Graphene Nanoribbons Using Alkyne Benzannulations. J. Am. Chem. Soc. 2016;138:9137–9144. doi: 10.1021/jacs.6b03014. [DOI] [PubMed] [Google Scholar]
- 68.Aguilar E., Sanz R., Fernández-Rodríguez M.A., García-García P. 1,3-Dien-5-ynes: Versatile Building Blocks for the Synthesis of Carbo- and Heterocycles. Chem. Rev. 2016;116:8256–8311. doi: 10.1021/acs.chemrev.6b00181. [DOI] [PubMed] [Google Scholar]
- 69.Carreras J., Gopakumar G., Gu L., Gimeno A., Linowski P., Petuškova J., Thiel W., Alcarazo M. Polycationic Ligands in Gold Catalysis: Synthesis and Applications of Extremely π-Acidic Catalysts. J. Am. Chem. Soc. 2013;135:18815–18823. doi: 10.1021/ja411146x. [DOI] [PubMed] [Google Scholar]
- 70.Stężycki R., Grzybowski M., Clermont G., Blanchard-Desce M., Gryko D.T. Z-Shaped Pyrrolo[3,2-b]pyrroles and Their Transformation into π-Expanded Indolo[3,2-b]indoles. Chem. Eur. J. 2016;22:5198–5203. doi: 10.1002/chem.201505052. [DOI] [PubMed] [Google Scholar]
- 71.Matsuda T., Moriya T., Goya T., Murakami M. Synthesis of Pyrenes by Twofold Hydroarylation of 2,6-Dialkynylbiphenyls. Chem. Lett. 2011;40:40–41. doi: 10.1246/cl.2011.40. [DOI] [Google Scholar]
- 72.Lorbach D., Wagner M., Baumgarten M., Müllen K. The Right Way to Self-fuse Bi- and Terpyrenyls to Afford Graphenic Cutouts. Chem. Commun. 2013;49:10578–10580. doi: 10.1039/c3cc45235b. [DOI] [PubMed] [Google Scholar]
- 73.Shen H.-C., Pal S., Lian J.-J., Liu R.-S. Ruthenium-Catalyzed Aromatization of Aromatic Enynes via the 1,2-Migration of Halo and Aryl Groups: A New Process Involving Electrocyclization and Skeletal Rearrangement. J. Am. Chem. Soc. 2003;125:15762–15763. doi: 10.1021/ja0379159. [DOI] [PubMed] [Google Scholar]
- 74.Lian J.-J., Odedra A., Wu C.-J., Liu R.-S. Ruthenium-Catalyzed Regioselective 1,3-Methylene Transfer by Cleavage of Two Adjacent σ-Carbon−Carbon Bonds: An Easy and Selective Synthesis of Highly Subsituted Benzenes. J. Am. Chem. Soc. 2005;127:4186–4187. doi: 10.1021/ja0504901. [DOI] [PubMed] [Google Scholar]
- 75.Fürstner A., Mamane V. Flexible Synthesis of Phenanthrenes by a PtCl2-Catalyzed Cycloisomerization Reaction. J. Org. Chem. 2002;67:6264–6267. doi: 10.1021/jo025962y. [DOI] [PubMed] [Google Scholar]
- 76.Mamane V., Hannen P., Fürstner A. Synthesis of Phenanthrenes and Polycyclic Heteroarenes by Transition-Metal Catalyzed Cycloisomerization Reactions. Chem. Eur. J. 2004;10:4556–4575. doi: 10.1002/chem.200400220. [DOI] [PubMed] [Google Scholar]
- 77.Donovan P.M., Scott L.T. Elaboration of Diaryl Ketones into Naphthalenes Fused on Two or Four Sides: A Naphthoannulation Procedure. J. Am. Chem. Soc. 2004;126:3108–3112. doi: 10.1021/ja038254i. [DOI] [PubMed] [Google Scholar]
- 78.Shen H.-C., Tang J.-M., Chang H.-K., Yang C.-W., Liu R.-S. Short and Efficient Synthesis of Coronene Derivatives via Ruthenium-Catalyzed Benzannulation Protocol. J. Org. Chem. 2005;70:10113–10116. doi: 10.1021/jo0512599. [DOI] [PubMed] [Google Scholar]
- 79.Chen T.-A., Lee T.-J., Lin M.-Y., Sohel S.M.A., Diau E.W.-G., Lush S.-F., Liu R.-S. Regiocontrolled Synthesis of Ethene-Bridged para-Phenylene Oligomers Based on PtII- and RuII-Catalyzed Aromatization. Chem. Eur. J. 2010;16:1826–1833. doi: 10.1002/chem.200902231. [DOI] [PubMed] [Google Scholar]
- 80.Shaibu B.S., Lin S.-H., Lin C.-Y., Wong K.-T., Liu R.-S. Ph2N-Susbtituted Ethylene-Bridged p-Phenylene Oligomers: Synthesis and Photophysical and Redox Properties. J. Org. Chem. 2011;76:1054–1061. doi: 10.1021/jo1020163. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe T., Abe H., Mutoh Y., Saito S. Ruthenium-Catalyzed Cycloisomerization of 2-Alkynylstyrenes via 1,2-Carbon Migration That Leads to Substituted Naphthalenes. Chem. Eur. J. 2018;24:11545–11549. doi: 10.1002/chem.201802413. [DOI] [PubMed] [Google Scholar]
- 82.Hertz V.M., Lerner H.-W., Wagner M. Ru-Catalyzed Benzannulation Leads to Luminescent Boron-Containing Polycyclic Aromatic Hydrocarbons. Org. Lett. 2015;17:5240–5243. doi: 10.1021/acs.orglett.5b02604. [DOI] [PubMed] [Google Scholar]
- 83.Wu T.-L., Chou H.-H., Huang P.-Y., Cheng C.-H., Liu R.-S. 3,6,9,12-Tetrasubstituted Chrysenes: Synthesis, Photophysical Properties, and Application as Blue Fluorescent OLED. J. Org. Chem. 2014;79:267–274. doi: 10.1021/jo402429q. [DOI] [PubMed] [Google Scholar]
- 84.Walker D.B., Howgego J., Davis A.P. Synthesis of Regioselectively Functionalized Pyrenes via Transition-Metal-Catalyzed Electrocyclization. Synthesis. 2010;2010:3686–3692. doi: 10.1055/s-0030-1258238. [DOI] [Google Scholar]
- 85.Liu J., Li B.-W., Tan Y.-Z., Giannakopoulos A., Sanchez-Sanchez C., Beljonne D., Ruffieux P., Fasel R., Feng X., Müllen K. Toward Cove-Edged Low Band Gap Graphene Nanoribbons. J. Am. Chem. Soc. 2015;137:6097–6103. doi: 10.1021/jacs.5b03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nijegorodov N.I., Downey W.S. The Influence of Planarity and Rigidity on the Absorption and Fluorescence Parameters and Intersystem Crossing Rate Constant in Aromatic Molecules. J. Phys. Chem. 1994;98:5639–5643. doi: 10.1021/j100073a011. [DOI] [Google Scholar]
- 87.Ionkin A.S., Marshall W.J., Fish B.M., Bryman L.M., Wang Y. A Tetra-substituted Chrysene: Orientation of Multiple Electrophilic Substitution and use of a Tetra-substituted Chrysene as a Blue Emitter for OLEDs. Chem. Commun. 2008:2319–2321. doi: 10.1039/b715386d. [DOI] [PubMed] [Google Scholar]
- 88.Eccleshare L., Selzer S., Woodward S. An Efficient Synthesis of Substituted Chrysenes. Tetrahedron Lett. 2017;58:393–395. doi: 10.1016/j.tetlet.2016.12.004. [DOI] [Google Scholar]
- 89.Rieger R., Kastler M., Enkelmann V., Müllen K. Entry to Coronene Chemistry—Making Large Electron Donors and Acceptors. Chem. Eur. J. 2008;14:6322–6325. doi: 10.1002/chem.200800832. [DOI] [PubMed] [Google Scholar]
- 90.Ghosh A., Rao K.V., George S.J., Rao C.N.R. Noncovalent Functionalization, Exfoliation, and Solubilization of Graphene in Water by Employing a Fluorescent Coronene Carboxylate. Chem. Eur. J. 2010;16:2700–2704. doi: 10.1002/chem.200902828. [DOI] [PubMed] [Google Scholar]
- 91.Hill J.P., Jin W., Kosaka A., Fukushima T., Ichihara H., Shimomura T., Ito K., Hashizume T., Ishii N., Aida T. Self-Assembled Hexa-peri-hexabenzocoronene Graphitic Nanotube. Science. 2004;304:1481–1483. doi: 10.1126/science.1097789. [DOI] [PubMed] [Google Scholar]
- 92.Stabel A., Herwig P., Müllen K., Rabe J.P. Diodelike Current–Voltage Curves for a Single Molecule–Tunneling Spectroscopy with Submolecular Resolution of an Alkylated, peri-Condensed Hexabenzocoronene. Angew. Chem. Int. Ed. 1995;34:1609–1611. doi: 10.1002/anie.199516091. [DOI] [Google Scholar]
- 93.Newman M.S. A New Synthesis of Coronene. J. Am. Chem. Soc. 1940;62:1683–1687. doi: 10.1021/ja01864a014. [DOI] [Google Scholar]
- 94.Jessup P., Reiss J. Cyclophanes. VII. Naphthalene and Phenanthrene Cyclophanes for the Synthesis of Coronene. Aust. J. Chem. 1977;30:843–850. doi: 10.1071/CH9770843. [DOI] [Google Scholar]
- 95.Lütke Eversloh C., Li C., Müllen K. Core-Extended Perylene Tetracarboxdiimides: The Homologous Series of Coronene Tetracarboxdiimides. Org. Lett. 2011;13:4148–4150. doi: 10.1021/ol201623f. [DOI] [PubMed] [Google Scholar]
- 96.Wu D., Zhang H., Liang J., Ge H., Chi C., Wu J., Liu S.H., Yin J. Functionalized Coronenes: Synthesis, Solid Structure, and Properties. J. Org. Chem. 2012;77:11319–11324. doi: 10.1021/jo302093t. [DOI] [PubMed] [Google Scholar]
- 97.Shen Y., Chen C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012;112:1463–1535. doi: 10.1021/cr200087r. [DOI] [PubMed] [Google Scholar]
- 98.Chen C.-F., Shen Y. Helicene Chemistry: From Synthesis to Applications. Springer; Berlin/Heidelberg, Germany: 2017. pp. 1–276. [Google Scholar]
- 99.Storch J., Sýkora J., Čermák J., Karban J., Císařová I., Růžička A. Synthesis of Hexahelicene and 1-Methoxyhexahelicene via Cycloisomerization of Biphenylyl-Naphthalene Derivatives. J. Org. Chem. 2009;74:3090–3093. doi: 10.1021/jo900077j. [DOI] [PubMed] [Google Scholar]
- 100.Storch J., Čermák J., Karban J., Císařová I., Sýkora J. Synthesis of 2-Aza[6]helicene and Attempts to Synthesize 2,14-Diaza[6]helicene Utilizing Metal-Catalyzed Cycloisomerization. J. Org. Chem. 2010;75:3137–3140. doi: 10.1021/jo100252a. [DOI] [PubMed] [Google Scholar]
- 101.Weimar M., Correa da Costa R., Lee F.-H., Fuchter M.J. A Scalable and Expedient Route to 1-Aza[6]helicene Derivatives and its Subsequent Application to a Chiral-Relay Asymmetric Strategy. Org. Lett. 2013;15:1706–1709. doi: 10.1021/ol400493x. [DOI] [PubMed] [Google Scholar]
- 102.Oyama H., Nakano K., Harada T., Kuroda R., Naito M., Nobusawa K., Nozaki K. Facile Synthetic Route to Highly Luminescent Sila[7]helicene. Org. Lett. 2013;15:2104–2107. doi: 10.1021/ol4005036. [DOI] [PubMed] [Google Scholar]
- 103.Oyama H., Akiyama M., Nakano K., Naito M., Nobusawa K., Nozaki K. Synthesis and Properties of [7]Helicene-like Compounds Fused with a Fluorene Unit. Org. Lett. 2016;18:3654–3657. doi: 10.1021/acs.orglett.6b01708. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka M., Shibata Y., Nakamura K., Teraoka K., Uekusa H., Nakazono K., Takata T., Tanaka K. Gold-Catalyzed Enantioselective Synthesis, Crystal Structure, and Photophysical/Chiroptical Properties of Aza[10]helicenes. Chem. Eur. J. 2016;22:9537–9541. doi: 10.1002/chem.201601622. [DOI] [PubMed] [Google Scholar]
- 105.Asao N., Nogami T., Lee S., Yamamoto Y. Lewis Acid-Catalyzed Benzannulation via Unprecedented [4+2] Cycloaddition of o-Alkynyl(oxo)benzenes and Enynals with Alkynes. J. Am. Chem. Soc. 2003;125:10921–10925. doi: 10.1021/ja036927r. [DOI] [PubMed] [Google Scholar]
- 106.Hein S.J., Lehnherr D., Arslan H., Uribe-Romo F.J., Dichtel W.R. Alkyne Benzannulation Reactions for the Synthesis of Novel Aromatic Architectures. Acc. Chem. Res. 2017;50:2776–2788. doi: 10.1021/acs.accounts.7b00385. [DOI] [PubMed] [Google Scholar]
- 107.Lehnherr D., Chen C., Pedramrazi Z., DeBlase C.R., Alzola J.M., Keresztes I., Lobkovsky E.B., Crommie M.F., Dichtel W.R. Sequence-defined Oligo(ortho-arylene) Foldamers Derived from the Benzannulation of Ortho(arylene ethynylene)s. Chem. Sci. 2016;7:6357–6364. doi: 10.1039/C6SC02520J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hein S.J., Lehnherr D., Dichtel W.R. Rapid Access to Substituted 2-Naphthyne Intermediates via the Benzannulation of Halogenated Silylalkynes. Chem. Sci. 2017;8:5675–5681. doi: 10.1039/C7SC01625E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arslan H., Walker K.L., Dichtel W.R. Regioselective Asao–Yamamoto Benzannulations of Diaryl Acetylenes. Org. Lett. 2014;16:5926–5929. doi: 10.1021/ol502938y. [DOI] [PubMed] [Google Scholar]
- 110.Arslan H., Saathoff J.D., Bunck D.N., Clancy P., Dichtel W.R. Highly Efficient Benzannulation of Poly(phenylene ethynylene)s. Angew. Chem. Int. Ed. 2012;51:12051–12054. doi: 10.1002/anie.201206964. [DOI] [PubMed] [Google Scholar]
- 111.Arslan H., Uribe-Romo F.J., Smith B.J., Dichtel W.R. Accessing Extended and Partially Fused Hexabenzocoronenes using a Benzannulation–Cyclodehydrogenation Approach. Chem. Sci. 2013;4:3973–3978. doi: 10.1039/c3sc51212f. [DOI] [Google Scholar]
- 112.Lehnherr D., Alzola J.M., Lobkovsky E.B., Dichtel W.R. Regioselective Synthesis of Polyheterohalogenated Naphthalenes via the Benzannulation of Haloalkynes. Chem. Eur. J. 2015;21:18122–18127. doi: 10.1002/chem.201503418. [DOI] [PubMed] [Google Scholar]
- 113.Lehnherr D., Alzola J.M., Mulzer C.R., Hein S.J., Dichtel W.R. Diazatetracenes Derived from the Benzannulation of Acetylenes: Electronic Tuning via Substituent Effects and External Stimuli. J. Org. Chem. 2017;82:2004–2010. doi: 10.1021/acs.joc.6b02840. [DOI] [PubMed] [Google Scholar]
- 114.Gao J., Uribe-Romo F.J., Saathoff J.D., Arslan H., Crick C.R., Hein S.J., Itin B., Clancy P., Dichtel W.R., Loo Y.-L. Ambipolar Transport in Solution-Synthesized Graphene Nanoribbons. ACS Nano. 2016;10:4847–4856. doi: 10.1021/acsnano.6b00643. [DOI] [PubMed] [Google Scholar]
- 115.Yamaguchi S., Swager T.M. Oxidative Cyclization of Bis(biaryl)acetylenes: Synthesis and Photophysics of Dibenzo[g,p]chrysene-Based Fluorescent Polymers. J. Am. Chem. Soc. 2001;123:12087–12088. doi: 10.1021/ja016692o. [DOI] [PubMed] [Google Scholar]
- 116.Yang W., Kazemi R.R., Karunathilake N., Catalano V.J., Alpuche-Aviles M.A., Chalifoux W.A. Expanding the scope of peropyrenes and teropyrenes through a facile InCl3-catalyzed multifold alkyne benzannulation. Org. Chem. Front. 2018;5:2288–2295. doi: 10.1039/C8QO00389K. [DOI] [Google Scholar]
- 117.Yang W., Monteiro J.H.S.K., de Bettencourt-Dias A., Catalano V.J., Chalifoux W.A. Synthesis, Structure, Photophysical Properties, and Photostability of Benzodipyrenes. Chem. Eur. J. 2018 doi: 10.1002/chem.201805248. [DOI] [PubMed] [Google Scholar]
- 118.Yang W., Bam R., Catalano V.J., Chalifoux W.A. Highly Regioselective Domino Benzannulation Reaction of Buta-1,3-diynes to Construct Irregular Nanographenes. Angew. Chem. Int. Ed. 2018;57:14773–14777. doi: 10.1002/anie.201808043. [DOI] [PubMed] [Google Scholar]
- 119.Seybold G., Wagenblast G. New Perylene and Violanthrone Dyestuffs for Fluorescent Collectors. Dyes Pigment. 1989;11:303–317. doi: 10.1016/0143-7208(89)85048-X. [DOI] [Google Scholar]
- 120.Gvishi R., Reisfeld R., Burshtein Z. Spectroscopy and Laser Action of the “Red Perylimide Dye” in Various Solvents. Chem. Phys. Lett. 1993;213:338–344. doi: 10.1016/0009-2614(93)85142-B. [DOI] [Google Scholar]
- 121.Franceschin M., Alvino A., Casagrande V., Mauriello C., Pascucci E., Savino M., Ortaggi G., Bianco A. Specific Interactions with Intra- and Intermolecular G-Quadruplex DNA Structures by Hydrosoluble Coronene Derivatives: A New Class of Telomerase Inhibitors. Biorg. Med. Chem. 2007;15:1848–1858. doi: 10.1016/j.bmc.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 122.An Z., Yu J., Domercq B., Jones S.C., Barlow S., Kippelen B., Marder S.R. Room-Temperature Discotic Liquid-Crystalline Coronene Diimides Exhibiting High Charge-Carrier Mobility in Air. J. Mater. Chem. 2009;19:6688–6698. doi: 10.1039/b910898j. [DOI] [Google Scholar]
- 123.Rohr U., Kohl C., Müllen K., van de Craats A., Warman J. Liquid Crystalline Coronene Derivatives. J. Mater. Chem. 2001;11:1789–1799. doi: 10.1039/b009708j. [DOI] [Google Scholar]
- 124.Müller S., Müllen K. Facile Synthetic Approach to Novel Core-Extended Perylene Carboximide Dyes. Chem. Commun. 2005:4045–4046. doi: 10.1039/b509220e. [DOI] [PubMed] [Google Scholar]
- 125.Bai Q., Gao B., Ai Q., Wu Y., Ba X. Core-Extended Terrylene Diimide on the Bay Region: Synthesis and Optical and Electrochemical Properties. Org. Lett. 2011;13:6484–6487. doi: 10.1021/ol202775b. [DOI] [PubMed] [Google Scholar]
- 126.Shao J., Zhao X., Wang L., Tang Q., Li W., Yu H., Tian H., Zhang X., Geng Y., Wang F. Synthesis and Characterization of π-Extended Thienoacenes with up to 13 Fused Aromatic Rings. Tetrahedron Lett. 2014;55:5663–5666. doi: 10.1016/j.tetlet.2014.08.073. [DOI] [Google Scholar]
- 127.Cheng S.-W., Tsai C.-E., Liang W.-W., Chen Y.-L., Cao F.-Y., Hsu C.-S., Cheng Y.-J. Angular-Shaped 4,9-Dialkylnaphthodithiophene-Based Donor–Acceptor Copolymers for Efficient Polymer Solar Cells and High-Mobility Field-Effect Transistors. Macromolecules. 2015;48:2030–2038. doi: 10.1021/acs.macromol.5b00098. [DOI] [Google Scholar]
- 128.Lai Y.-Y., Chang H.-H., Lai Y.-Y., Liang W.-W., Tsai C.-E., Cheng Y.-J. Angular-Shaped 4,10-Dialkylanthradiselenophene and Its Donor–Acceptor Conjugated Polymers: Synthesis, Physical, Transistor, and Photovoltaic Properties. Macromolecules. 2015;48:6994–7006. doi: 10.1021/acs.macromol.5b01541. [DOI] [Google Scholar]
- 129.Lee C.-H., Lai Y.-Y., Cao F.-Y., Hsu J.-Y., Lin Z.-L., Jeng U.S., Su C.-J., Cheng Y.-J. Synthesis, Molecular and Photovoltaic/Transistor Properties of Heptacyclic Ladder-type Di(thienobenzo)fluorene-based Copolymers. J. Mater. Chem. C. 2016;4:11427–11435. doi: 10.1039/C6TC04300C. [DOI] [Google Scholar]