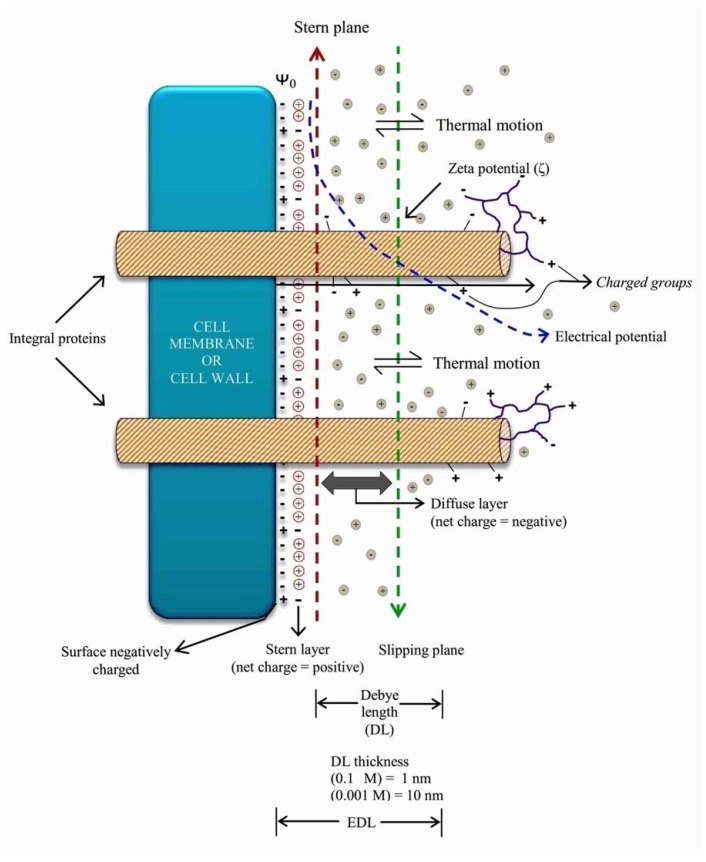
Figure 1.
Schematic representation of the electric double layer (EDL) as a dynamic structure on the surface of a charged cell wall or membrane of a living cell, nanoparticle (NP) or a nanomaterial (NM) when it is exposed to a fluid. The term EDL refers to two parallel layers of charge on the surface. The Stern layer (with a positive net charge), consists of ions adsorbed onto the surface due to chemical interactions. The diffuse layer (with a negative net charge) is composed of ions attracted to the surface charges of the Stern layer via the Coulomb force. The diffuse layer is made of free ions that move in the fluid under the influence of electric attraction and thermal motion. The Debye length is the thickness of the mobile ions of the EDL and marks the distance under the influence of the electric potential of the surface. The zeta potential is the electrical potential at the slipping plane. The volume contained under the slipping plane shows tangential molecular motion with respect to the surface. In diluted solutions (0.001 M) the Debye length is about 10 nm but decreases with the ionic strength (1 nm with 0.1 M). In plants, the Debye length is within 1–2 nm, because in biological fluids the ionic strength is often about 0.15 M [20]. Since the transmembrane domains of integral proteins can protrude from 2 to 7 nm [21], groups with positive and negative charges of proteins and recognition sites or receptors are located outside the EDL. On the other hand, the peripheral proteins associated with integral proteins are located even further away from the EDL.