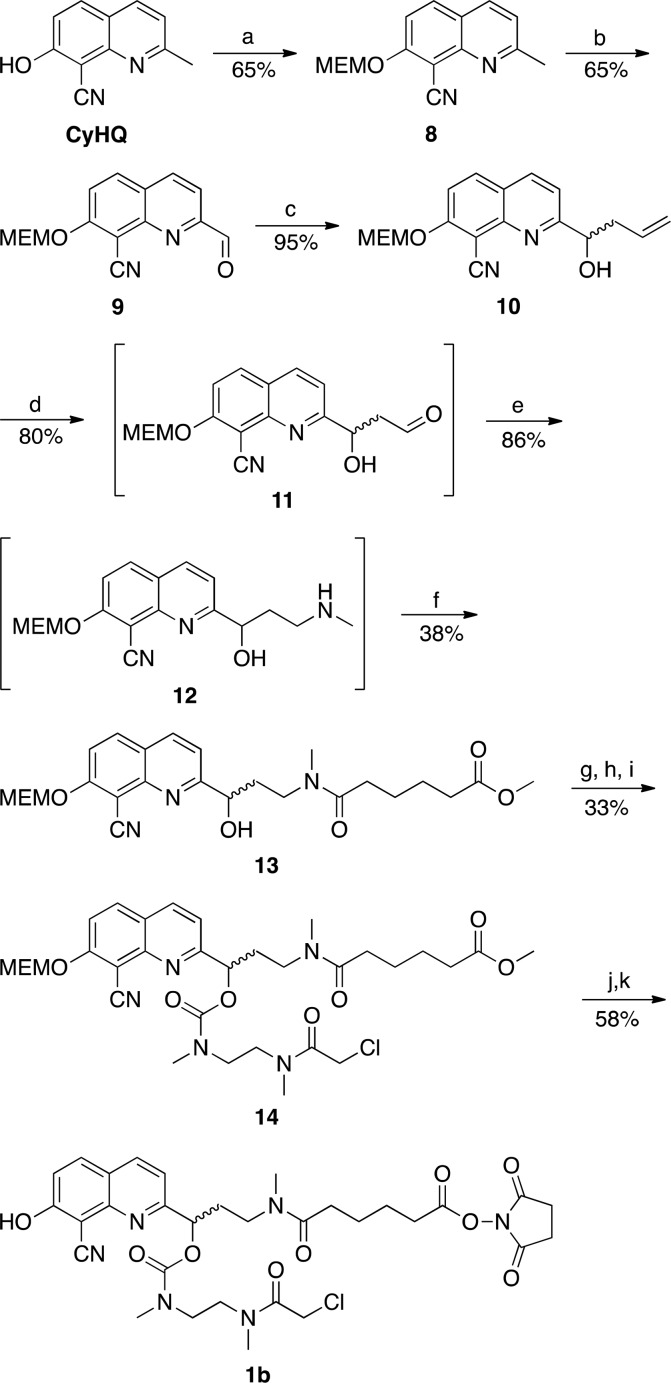
Scheme 2. Synthesis of CyHQ Linker 1ba.
Reagents and conditions: (a) MEMCl, iPr2NEt, CH2Cl2, 3 h; (b) SeO2, t-Bu-OOH, 1,4-dioxane, 45 °C, 3 h; (c) allyl bromide, indium, sat. NH4Cl(aq), THF, sonication, 55 °C, 90 min; (d) K2OsO4, 2,6-lutidine, NaIO4, 1:1 THF/H2O, 12 h; (e) methylamine (2.0 M in MeOH), AcOH, NaBH(OAc)3, THF, 12 h; (f) methyl adipoyl chloride, CH2Cl2, DIEA, 0 °C to rt, 6 h; (g) carbonyl diimidazole, CH2Cl2, 10 min; (h) N,N′-dimethylethylenediamine, CH2Cl2, 0 °C, 10 min; (i) chloroacetyl chloride, DIEA, 0 °C, 4 h; (j) trifluoroacetic acid/water (1:1), 40 °C, 12 h; (k) disuccinimidyl carbonate, pyridine, CH3CN, 12 h.