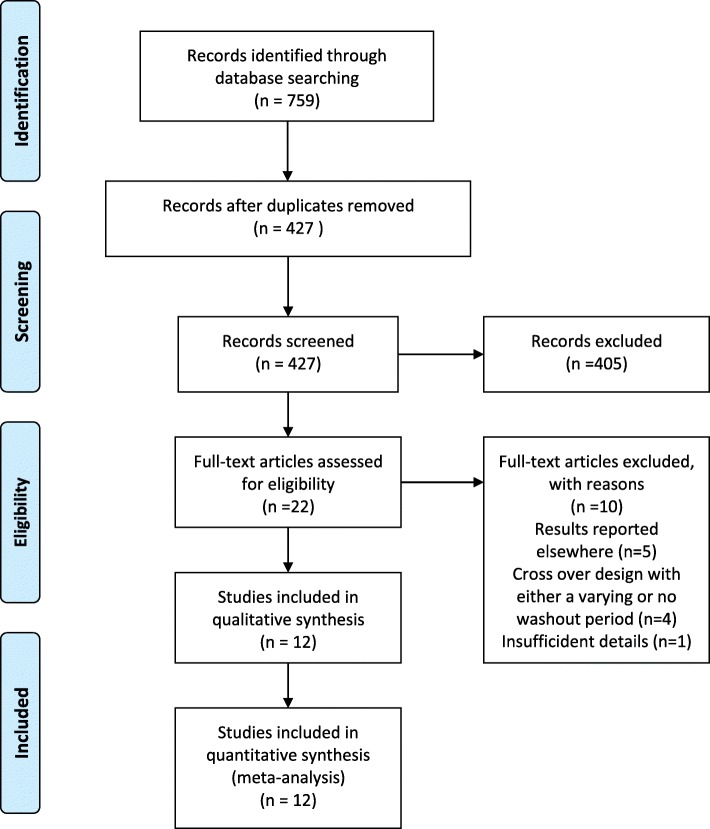
Fig. 1.
PRISMA Flow Diagram for Study Selection. PRISMA flowchart illustrating the process for the selection of the included articles for the systematic review and for the data synthesis of the randomized controlled clinical trials of enteric-coated peppermint oil versus placebo in patients with irritable bowel syndrome (IBS). Inclusion: Adult patients (18 years or greater) with IBS as diagnosed using any of the following criteria for IBS: Manning Criteria, Rome I, II, III, IV diagnostic criteria who were randomized to enteric-coated peppermint oil or placebo for a minimum of two weeks. Exclusion: Patients having an organic disease or who had not had an organic disease were excluded. Non-randomized trials; observational studies such as cohort study, cross-sectional study, etc.. A detailed evaluation of the articles by at least two independent reviewers (total of three) assessed the sufficiency of data and relevance to the topic. Seven hundred and fifty-nine articles were identified using PubMed (n = 102)/EMBASE (n = 396)/Cochrane (n = 60)/Web of Science (n = 201) search engines. After de-duplication, 427 records were screened and 22 deemed suitable for full-text review. Ten articles were eliminated, thus leaving twelve for qualitative and data synthesis