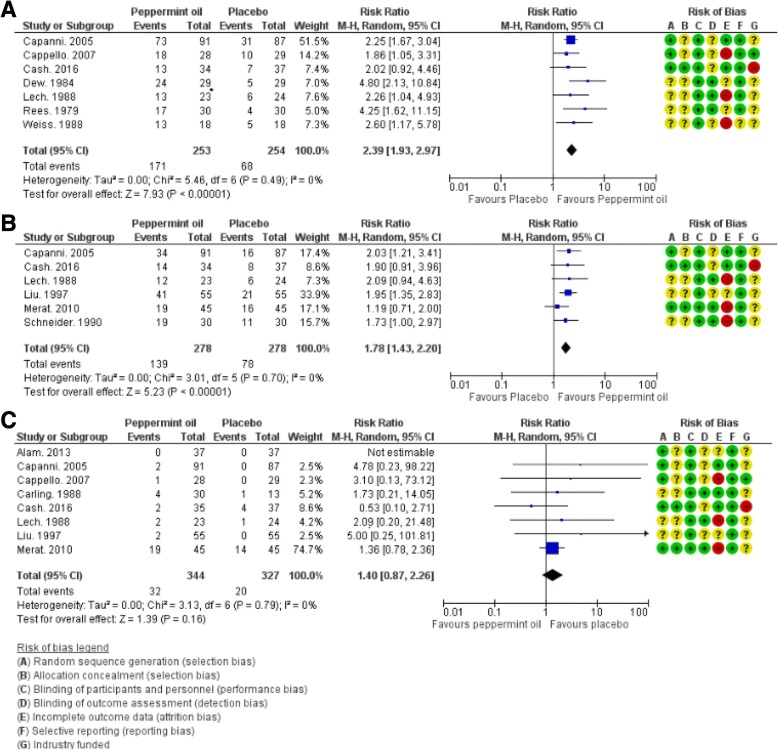
Fig. 3.
a-c. Forrest Plots of Meta-analysis of Enteric-Coated Peppermint Oil for the treatment of Irritable Bowel Syndrome. The results of our meta-analysis of the randomized controlled trials of enteric-coated peppermint oil (PO) versus placebo for the irritable bowel syndrome (IBS) are shown in a-c. a illustrates the results of the meta-analysis of treatment outcomes for seven included studies for the global improvement of IBS symptoms vs. placebo [20, 22, 31, 33–35, 37]. a. illustrates the risk ratio (RR) for seven RCTs for the effect of enteric-coated PO (n = 253) versus placebo (n = 254) on global symptoms was 2.39 [95% confidence interval (CI): 1.93, 2.97], I2 = 0%, z = 7.93 (p < 0.00001). b. displays the results of the meta-analysis of the reported treatment outcomes for the improvement in abdominal pain vs. placebo for six included studies [22, 31, 32, 34, 36]. The RR for six RCTs for the effect of enteric-coated PO (n = 278) versus placebo (n = 278) on abdominal pain was 1.78 [95% CI: 1.43, 2.20], I2 = 0%, z = 5.23 (p < 0.00001). c illustrates the results of the meta-analysis of eight included studies [22, 29–32, 34, 35, 38] of the reported adverse effects in IBS subjects using EPO (32 events, 344 total, 9.3%) versus placebo (20 events, 327 total, 6.1%); RR 1.40 [95% CI: 0.87, 2.26] I2 = 0%, z = 1.39 (p = 0.16). Figure 3a-c show their corresponding risk of bias analysis. (A) Random sequence generation (selection bias); (B) Allocation concealment (selection bias); (C) Blinding of participants and personnel (performance bias); (D) Blinding of outcome assessment (detection bias); (E) Incomplete outcome data (attribution bias); (F) Selective reporting (reporting bias); (G) Industry funded. The risk of bias is displayed as low risk (green, +), unclear (yellow, ?), or high risk (red, −)