Abstract
We aimed to identify demographic and clinical predictors of varicocele repair in a contemporary cohort of men in the USA. We queried the 2009–2015 MarketScan Database using relevant ICD9, ICD10, and CPT codes to identify all 18–45 year olds with varicoceles. Differences in age, area of residence, clinical characteristics, and medical management between men who did and did not undergo varicocelectomy (open, laparoscopic, or microsurgical) during the study period were compared using unpaired t-tests and Chi-squared tests for continuous and categorical variables, respectively. Multivariable logistic regression analysis was used to evaluate age, semen analyses, and serum hormone assessment as predictors of varicocele repair. SAS version 9.4 was used for all statistical analyses. Significance was set at P < 0.05. Approximately 40% of men with varicoceles underwent repair, primarily through an open approach. Men who underwent repair were more likely to have a diagnosis of male infertility (15.5% vs 7.9%, P < 0.001) and male hypogonadism (3.4% vs 0.9%) and were more likely to complete semen analyses (36.1% vs 12.2%, P < 0.001) and serum testosterone evaluation (42.5% vs 18.8%, P < 0.001). In multivariable regression models, the strongest predictors of varicocele repair were semen analysis (OR = 2.78, 95% CI: 2.56–3.02), age 18–25 years (OR = 2.66, 95% CI: 2.36–2.98), and serum testosterone evaluation (OR = 1.67, 95% CI: 1.51–1.86). Although male infertility remains the most important indication for varicocele repair, male hypogonadism is emerging as an independent predictor of varicocelectomy, which may represent a change in the clinical management of varicoceles in the USA.
Keywords: decision-making, hypogonadism, male infertility, varicocele, varicocelectomy
INTRODUCTION
Varicoceles affect 15%–20% of the general male population and up to 40% of infertile men.1 Varicoceles have known deleterious effects on both spermatogenic and steroidogenic functions of the testis and remain the most common correctable cause of male factor infertility.2 The American Urological Association Best Practice Policy and the American Society of Reproductive Medicine Practice Committee Report on Varicocele and Infertility specifically outline indications for the diagnostic workup and treatment of varicoceles, but variations in diagnosis and management still exist.1,3
There is increasing evidence documenting an association between low serum testosterone levels and the presence of varicoceles.4 Although several analyses have demonstrated an improvement in serum testosterone levels following varicocele repair, the majority of these studies have been observational. Conclusive evidence supporting definitive and long-term improvement of testosterone levels after varicocelectomy is lacking,5,6,7,8 and hypogonadism is not considered an indication for varicocele repair according to the current clinical guidelines.1,3 Multiple studies show that microsurgical technique is superior to laparoscopic and open repairs because of better reproductive outcomes and lower incidence of postoperative complications and recurrence.9,10,11 Nevertheless, microsurgical approach to varicocele repair has not been universally adopted to date.
Our study aimed to investigate contemporary national trends in the diagnosis and management of varicoceles in order to identify the most important predictors of varicocele repair.
METHODS
We performed a cross-sectional data analysis of the MarketScan Commercial Claims and Encounters Database, which contains information from individual-level health-care claims on more than 13 million insured Americans covered by employer-sponsored, private health insurance.8 Relevant CPT, ICD9, and ICD10 codes were used to identify all 18–45-year-old men diagnosed with a varicocele during 2009–2015 (Supplementary Table 1 (561.6KB, tif) and 2 (111.2KB, tif) ). Differences in age, clinical characteristics, and medical management between men who did and did not undergo varicocele repair during the study period were compared using unpaired t-tests and Chi-squared tests for continuous and categorical variables, respectively. Surgical approach to varicocele repair was stratified by geographic location inside or outside a metropolitan statistical area. Multivariable logistic regression analysis was used to evaluate age, semen parameters, and serum hormone levels as predictors of varicocele repair, based on claims associated with semen analyses, blood hormone tests, prescriptions for hormone therapies, and diagnosis codes for male infertility and male hypogonadism. Distribution of health insurance plans between men who did and did not undergo varicocele repair was also examined. SAS version 9.4 (SAS Institute Incorporated, Cary, NC, USA) was used for all statistical analyses. Significance was set at P < 0.05.
Summary of Current Procedural Terminology codes used in the analysis
Summary of International Classification of Diseases 9 and International Classification of Diseases 10 codes used in the analysis
Because we did not expect providers to consistently enter more than one diagnosis code, we relied on billing codes for semen analyses, serum testosterone (T), and follicle-stimulating hormone (FSH) to comprehensively capture the diagnostic workup for men with varicoceles. Since serum T testing is usually a component of the male infertility evaluation, we specifically investigated the frequency of male infertility- and male hypogonadism-related diagnoses in the study population, as well as the frequency with which FSH levels were evaluated in this cohort of men. We assumed that men undergoing concomitant T and FSH testing were likely undergoing evaluation for male factor infertility. We also assumed that men undergoing T testing only were being evaluated for hypogonadism, related to varicocele or otherwise.
This study was approved by the Emory University Institutional Review Board. This secondary data analysis of a de-identified population-level dataset did not require informed consent from individual patients.
RESULTS
Table 1 summarizes the demographic and clinical characteristics of the 21 195 men diagnosed with varicoceles between 2009 and 2015. Of these, 8231 men (38.8%) underwent surgical repair. Surgery was performed using either an open (82.0%), microsurgical (10.9%), or laparoscopic (7.1%) approach. The use of radiographic embolization was rare (<0.5%, data not reported). The approach to surgical repair did not differ between metropolitan statistical area and rural area.
Table 1.
Summary of demographic, geographic, and clinical characteristics of US men diagnosed with varicoceles
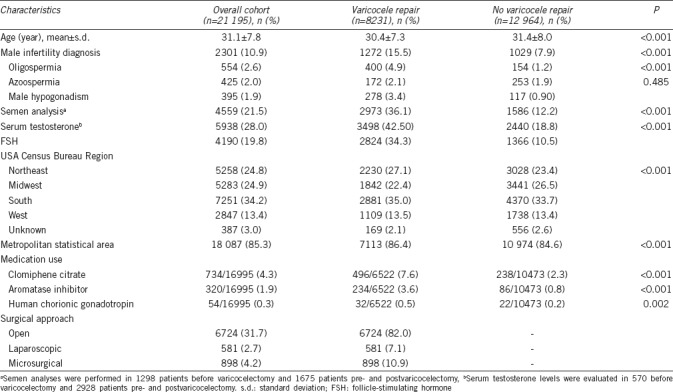
The mean age (mean ± standard deviation [s.d.]) of men who underwent varicocele repair was similar to those who did not undergo varicocele repair (30.4 ± 7.3 years vs 31.4 ± 8.0 years) (Table 1). Men who underwent varicocele repair were more likely to have a diagnosis of male infertility (15.5% vs 7.9%, P < 0.001), oligospermia (4.9% vs 1.2%, P < 0.001), and hypogonadism (3.4% vs 0.9%, P < 0.001) compared to those who did not undergo repair. In terms of clinical evaluation, men who underwent varicocele repair were more likely to undergo semen analyses (36.1% vs 12.2%, P < 0.001) and serum testosterone evaluation (42.5% vs 18.8%, P < 0.001) compared to those who did not undergo repair. Approximately 5% of the overall cohort of men used nontestosterone-based hormonal therapy. Interestingly, the use of gonadotropin therapy (0.49% vs 0.21%, P = 0.002), selective estrogen receptor modulators (7.6% vs 2.3%, P < 0.001), and aromatase inhibitors (3.6% vs 0.8%, P < 0.001) was more common among men who underwent varicocele repair compared to those who did not.
In multivariable regression models, the strongest predictors of varicocele repair were semen analysis evaluation (OR = 2.78, 95% CI: 2.56–3.02), age 18–25 years (OR = 2.66, 95% CI: 2.36–2.98), hypogonadism diagnosis (OR = 2.00, 95% CI: 1.57–2.55), and serum testosterone evaluation (OR = 1.67, 95% CI: 1.51–1.86) (Table 2). Other significant indicators of varicocele repair included the use of hormone therapies, such as clomiphene citrate, aromatase inhibitors, and gonadotropins (OR = 1.32, 95% CI: 1.13–1.55), and male infertility diagnosis (OR = 1.29, 95% CI: 1.16–1.42). These results are summarized in Table 2.
Table 2.
Multivariable regression analysis of predictors of varicocele repair
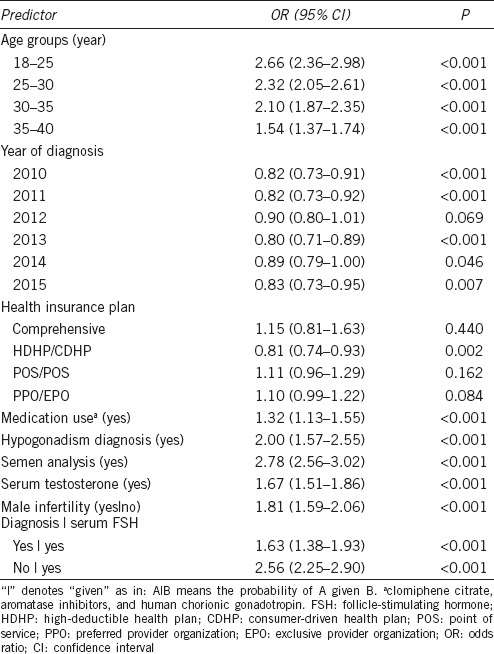
Only 395 patients in the overall cohort of 21 195 men had a diagnosis of male hypogonadism. Of men who underwent varicocele repair, 42.5% underwent testosterone evaluation alone, while 33.3% underwent both testosterone and FSH evaluation. Even after controlling for FSH evaluation in the multivariable regression model, testosterone evaluation was still a significant predictor of varicocele repair (OR = 1.67, 95% CI: 1.51–1.86).
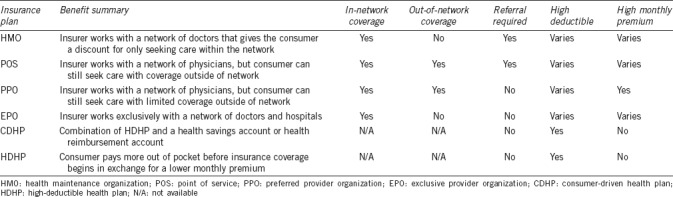
The different health plans represented in the study cohort were grouped based on plan description (Table 3). Men with high-deductible health plans/consumer-driven health plans (HDHP/CDHP) were less likely to undergo varicocele repair compared to health maintenance organization (HMO) plans (OR = 0.81, 95% CI: 0.74–0.93), while comprehensive and network plans, i.e., point of service (POS/POS with cap) and preferred provider organization/exclusive provider organization (PPO/EPO) plans, had no significant association with varicocele repair (Table 2).
Table 3.
Summary of comprehensive health plans
DISCUSSION
This population-level analysis of trends in the diagnosis and management of varicoceles demonstrates that open varicocelectomy remains the most common approach to varicocele repair, despite considerable evidence identifying microsurgical varicocelectomy as the safest and most effective method of varicocele repair.4,9 Male infertility was the most important indication for varicocele repair, which is consistent with the current American Urological Association (AUA) practice recommendations. However, male hypogonadism was found to be an independent predictor of varicocelectomy, which may represent an emerging and evolving change in the management of varicoceles in the USA.
Several studies have demonstrated a connection between low testosterone levels and varicoceles, presumably due to the detrimental effect of varicoceles on testicular function. Some studies have shown a mean improvement in serum testosterone levels of approximately 100 ng dl−1 after varicocelectomy.5,6,7,8 These studies have largely been observational in nature, with limited number of patients and limited follow-up. It is unclear whether improvement in T levels associated with varicocelectomy is reliable or sustainable over time. It is also unclear if the magnitude of improvement in T levels following varicocele repair would be sufficient to prevent patients from requiring testosterone-replacement therapy in the long term. Finally, there is a potential for publication bias with respect to this question, as studies showing no improvement in serum T levels following varicocelectomy may be less likely to be published. For all these reasons, low serum T levels are not currently considered a clinical indication for varicocele repair. While our results demonstrate a trend toward the use of hypogonadism as a predictor of varicocele repair, the design of this study does not permit evaluation of the utility of hypogonadism as a clinical indication for varicocelectomy.
Discrepancies in the association between health insurance plans and varicocele repair was an interesting finding. One possible explanation for the significant negative correlation between high-deductible health plans (HDHP/CDHP) and varicocele repair is the large out-of-pocket cost for the patient. In contrast, comprehensive and network plans (POS, EPO, PPO, and HMO) generally have lower deductibles and less out-of-pocket cost. According to the USA Internal Revenue Service, a high-deductible health plan is any plan with a deductible of at least $1300 for an individual or $2600 for a family.12 Patients with high-deductible plans would likely have to pay for most, if not all, of the surgery, which has been estimated to cost $2248.21 for a microsurgical repair and $1617.39 for a nonmicrosurgical repair.5 An association has been demonstrated between low-income families with HDHPs and a cost-related delay or omission of care, when compared to high-income patients with similar plans.13 This association could plausibly explain the trend, as the high out-of-pocket cost may deter a portion of patients with these health plans from seeking medical care and opting for surgery.
The high proportion of open varicocelectomies is significant, given several studies showing its inferiority to microsurgical technique. We speculate that this trend could be due to several physician-level issues. First, physicians may not be adequately trained in microsurgery. Microsurgical technique was first introduced in 1985 by Marmar et al.14 and further modified in 1992 by Goldstein et al.15 It was not until recent years, though, that the substantial advantages of microsurgical technique in paternity rates, postoperative complications, and recurrence rates have been studied and confirmed.9,10,16 However, it is a more challenging operation than open repair for several reasons, including the greater number of vessels confronted at a microsurgical level and adjusting to the use of the microscope, which may partially explain the preference for open repair. Second, many facilities may not have access to operating microscopes. This may be due to the high upfront cost of a microscope, even though microsurgical repair has been established as the most cost-effective method for treating varicocele-associated causes for male infertility.17 Finally, physicians may prefer open over microsurgical repair due to the shorter operating times. Given the ever-increasing demands of physicians, the ability to complete more operations in a shorter amount of time is a notable advantage that may be driving this trend toward open repair. This finding could also be due to an error in coding and billing, as an additional billing code is needed to signify that a microsurgical approach was used. Notably, the use of radiographic embolization in this cohort was rare, which likely represents a decline in the use of this approach in the USA. Indeed, in recent years, publications discussing the use of radiographic embolization have been primarily based on non-USA cohorts.
We utilized a large, population-based cohort of patients to evaluate clinical practice patterns at a national level. This allowed us to make conclusions that are more generalizable when compared to the smaller, institutional cohorts that have been studied previously. That said, our study does have limitations, many of which are inherent to the retrospective nature of this study. First, we utilized a commercial claim dataset, which excluded patients without private insurance, and missed services that were not billed through insurance. Second, we searched within the dataset using specific CPT, ICD9, and ICD10 codes. Although we attempted to accurately capture each procedure and laboratory, variations in coding and billing certainly exist between providers and health-care systems and could affect our outcomes. A third limitation is that we did not have access to specific laboratory values and were left to make assumptions about medical decision-making about why patients did or did not undergo varicocele repair. Finally, this commercial claims dataset did not provide any information about varicoceles being unilateral or bilateral, or any information on varicocele size.
We recognize that for coding and billing purposes, patients with varicoceles who are being evaluated and treated for male infertility may be diagnosed with varicocele alone or varicocele and hypogonadism, since insurance coverage for male infertility diagnoses is inconsistent. Therefore, in addition to analysis diagnosis codes, we specifically evaluated the proportion of patients undergoing serum testosterone evaluation for T and FSH, in an effort to truly distinguish those undergoing infertility evaluation from those undergoing evaluation for hypogonadism. It should be emphasized that although our analysis demonstrated an independent association between infertility, hypogonadism, and varicocele repair, our study was retrospective in nature. A prospective study, which includes a comprehensive assessment of why men do or do not undergo varicocele repair, is needed to validate these findings.
AUTHOR CONTRIBUTIONS
CG helped conceive the study, interpreted the results, and drafted the manuscript. DP performed the data analysis and helped with interpretation of the results and manuscript review. AM helped conceive the study, provided oversight for data analysis and interpretation, and contributed to manuscript preparation. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by a Urology Care Foundation Research Scholar Award (AM).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.American Urological Association. Report on varicocele and infertility: best practice policy. Fertil Steril. 2001;82(Suppl 1):S142–5. doi: 10.1016/j.fertnstert.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 2.Neto F, Bach P, Najari B, Li P, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. doi: 10.1016/j.semcdb. 2016.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Shridharani A, Owen RC, Elkelany OO, Kim ED. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl. 2016;18:269–75. doi: 10.4103/1008-682X.172641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomboy J, Coward R. The varicocele: clinical presentation, evaluation, and surgical management. Semin Intervent Rad. 2016;33:163–9. doi: 10.1055/s-0036-1586143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Meguid T, Farsi H, Al-Sayyad A, Tab A, Mossi H, et al. Effects of varicocele and serum testosterone and changes of testosterone after varicocelectomy: a prospective controlled study. Urology. 2014;84:1081–7. doi: 10.1016/j.urology.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao W, Rosoff J, Pale J, Powell J, Goldstein M. Varicocelectomy is associated with increases in serum testosterone independent of clinical grade. Urology. 2013;81:1213–8. doi: 10.1016/j.urology.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Tanrikut C, Goldstein M, Rosoff J, Lee R, Nelson C, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 2011;108:1480–4. doi: 10.1111/j.1464-410X.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Yue H, Yamaguchi K, Okada K, Matsushita K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol. 2012;19:149–54. doi: 10.1111/j.1442-2042.2011.02890.x. [DOI] [PubMed] [Google Scholar]
- 9.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Duan X, Yang X, Deng X, Cui S. Laparoendoscopic single-site varicocelectomy compared with conventional laparoscopic surgery: a systematic review and meta-analysis. Springer Plus. 2016;5:1483. doi: 10.1186/s40064-016-3178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan R, Zhuo H, Cao D, Wei Q. Efficacy and safety of varicocelectomies: a meta-analysis. Syst Biol Reprod Med. 2017;63:120–9. doi: 10.1080/19396368.2016.1265161. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Centers for Medicaid and Medicare Services. Health Insurance Plan and Network Types: HMOs, PPOs, and More. [Last accessed on 2018 Jan 03]. Available from: http://www.github.ea-archive.org/ healthcare.gov/can-i-keep-my-own-doctor.html .
- 13.Kullgren JT, Galbraith AA, Hinrichsen VL, Miroshnik I, Penfold RB, et al. Health care use and decision making among lower-income families in high-deductible health plans. Arch Intern Med. 2010;170:1918–25. doi: 10.1001/archinternmed.2010.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmar JL, DeBenedictis TJ, Praiss D. The management of varicoceles by microdissection of the spermatic cord at the external inguinal ring. Fertile Steril. 1985;43:583–8. doi: 10.1016/s0015-0282(16)48501-8. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148:1808–11. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 16.Adamson D, Chang S, Hansen L. Health Research Data for the Real World: The MarketScan Databases. Thomson Healthcare. 2008 [Google Scholar]
- 17.Kovac JR, Fantus J, Lipshultz LI, Fischer MA, Klinghoffer Z. Cost-effectiveness analysis reveals microsurgical varicocele repair is superior to percutaneous embolization in the treatment of male infertility. Can Urol Assoc J. 2014;8:619–25. doi: 10.5489/cuaj.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of Current Procedural Terminology codes used in the analysis
Summary of International Classification of Diseases 9 and International Classification of Diseases 10 codes used in the analysis


