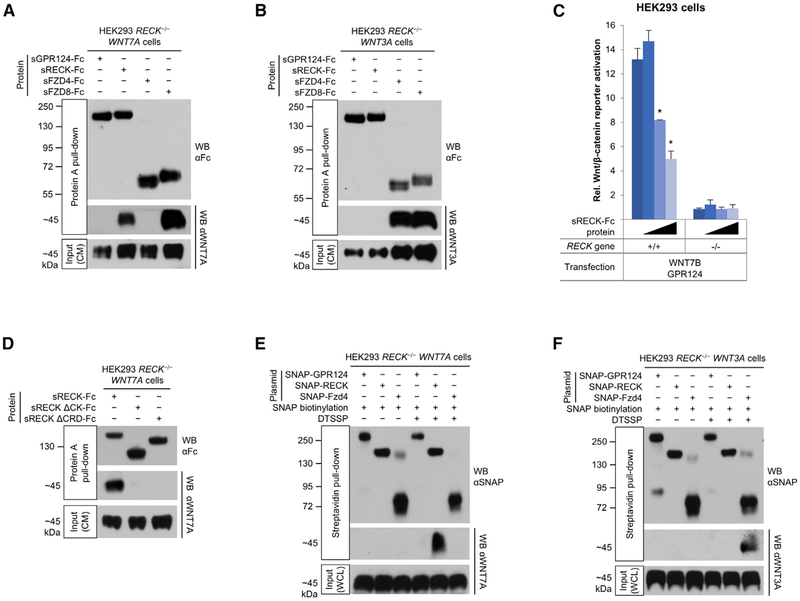
Figure 2. RECK Is a WNT7 Receptor.
(A) WNT7A binds to RECK and FZD8. HEK293 RECK−/− WNT7A cells were cultured in medium supplemented with indicated purified proteins (25 nM) for 72 hr and CM subjected to Protein A pull-down.
(B) WNT3A binds to FZD4 and FZD8, but not to GPR124 and RECK. HEK293 RECK−/− WNT3A cells were cultured in medium supplemented with indicated purified proteins (25 nM) for 72 hr and CM subjected to Protein A pull-down.
(C) Super TOP-Flash (STF) canonical Wnt/β-catenin reporter gene assay. HEK293 RECK+/+ or RECK−/− cells were co-transfected with the indicated expression constructs and STF/RLuc reporters. 24 hr after transfection sRECK-Fc protein was added at 0, 10, 100, or 1,000 nM (wedges). 24 hr later STF activity was normalized with RLuc activity. Mean (n = 3) ± SD. *p < 0.05 versus no protein.
(D) WNT7A binding to RECK is mediated by RECK cystine knot motif (CK) and cysteine-rich domains (CRD). HEK293 RECK−/− WNT7A cell culture medium was supplemented with the indicated proteins (3 nM) and conditioned for 96 hr. CM were subjected to Protein A pull-down.
(E and F) Cell surface RECK binds to WNT7A, but not to WNT3A. HEK293 RECK−/− WNT7A (E) or WNT3A (F) cells were transfected with the indicated expression constructs. 48 hr after transfection, SNAP tags were specifically biotinylated (bSNAP) and cell surface proteins cross-linked in situ using DTSSP (100 μM). Cells were lysed, subjected to streptavidin pull-down, and cross-links reversed. WCL, whole cell lysate.
All western blots (WB) were performed under reducing conditions. All data are representative of at least two independent experiments with similar results. CM, conditioned medium; s, soluble ECD; Fc, IgG Fc fragment.