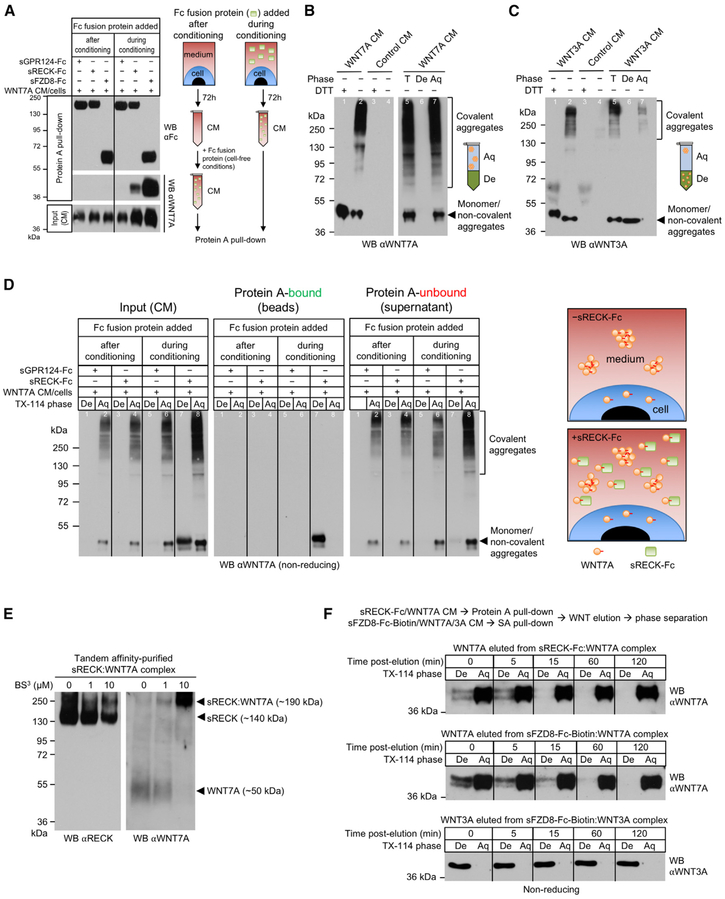
Figure 3. RECK Binding Stabilizes Short-Lived, Active, Monomeric, Hydrophobic WNT7A.
(A) RECK and FZD8 only bind newly secreted WNT7A. Culture media of HEK293 RECK−/− WNT7A cells were supplemented with the indicated proteins (100 nM) and conditioned for 72 hr (“during conditioning”). Alternatively, the indicated proteins were added to the CM after harvesting after 72h (“after conditioning”). CM were subjected to Protein A pull-down.
(B and C) CM from HEK293 RECK−/− WNT7A (B) or WNT3A (C) cells were analyzed by reducing (+DTT) or non-reducing (−DTT) SDS-PAGE/WB. Where indicated CM were subjected to Triton X-114 phase separation.
(D) sRECK-Fc protein stabilizes newly secreted, monomeric, hydrophobic WNT7A. Culture medium of HEK293 RECK−/− WNT7A cells was supplemented with indicated proteins (100 nM) and conditioned for 72 hr (“during conditioning”). Alternatively, indicated proteins were added to CM after harvesting after 72 hr (“after conditioning”). CM aliquots were subjected to Triton X-114 phase separation and remaining CM to Protein A pull-down followed by Triton X-114 phase separation of pull-downs and unbound supernatants. Irrelevant lanes (between lanes 4 and 5) have been removed from the WB.
(E) RECK and WNT7A form a 1:1 complex. Expi293F cells were co-transfected with sRECK-His and WNT7A. 96 hr after transfection CM was harvested and sRECK-His:WNT7A complex purified by combined chemical cross-linking (BS3)/tandem affinity purification. Purified complex was analyzed by reducing SDS-PAGE/WB.
(F) Free, hydrophobic WNT7A is highly unstable. sRECK-Fc:WNT7A, sFZD8-Fc-Biotin:WNT7A, and sFZD8-Fc-Biotin:WNT3A complexes were isolated from the corresponding HEK293 RECK−/− WNT7A/WNT3A CM (96 hr, 100 nM recombinant protein) using Protein A or streptavidin agarose. Bound Wnt proteins were eluted by low pH, neutralized, diluted into PBS/10% FBS and incubated at 37°C. At indicated time points Wnt eluate was subjected to Triton X-114 phase separation.
All data are representative of at least two independent experiments with similar results. CM, conditioned medium; WB, western blot; T, total; De, detergent phase; Aq, aqueous phase; s, soluble ECD; Fc, IgG Fc fragment.