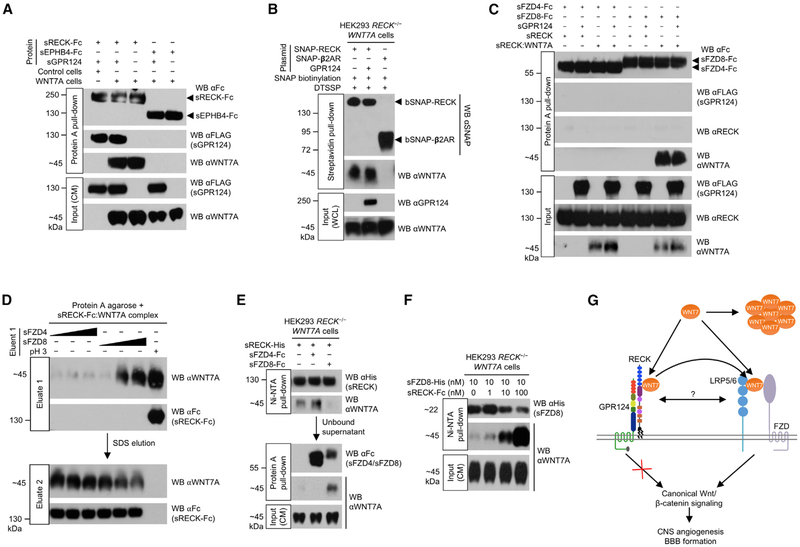
Figure 4. RECK Promotes FZD8:WNT7A Complex Formation.
(A) GPR124, RECK, and WNT7A form a ternary complex. HEK293 RECK−/− (control) or HEK293 RECK−/− WNT7A cells were cultured in medium supplemented with the indicated proteins (50 nM) for 72 hr. CM were harvested and subjected to Protein A pull-down.
(B) GPR124 does not regulate cell surface RECK:WNT7A complex formation. Cells were transfected with indicated expression constructs for 48 hr. SNAP tags were specifically biotinylated (bSNAP) and cell surface proteins cross-linked in situ by DTSSP (100 μM). Cells were lysed, subjected to streptavidin pull-down, and cross-links reversed. WCL, whole cell lysate. β2AR, β2-adrenergic receptor.
(C) FZD/GPR124/RECK/WNT7A quaternary complex formation is not detectable in vitro. sRECK-His:WNT7A complex was purified from HEK293 RECK−/− WNT7A CM (72 hr, 100 nM sRECK-His) using Ni-NTA agarose. Indicated purified proteins/sRECK:WNT7A complex (1 μM each) were allowed to form complexes and subjected to Protein A pull-down. Faint non-specific sRECK binding in all lanes.
(D) FZD8 elutes WNT7A from RECK. sRECK-Fc:WNT7A complex was isolated from HEK293 RECK−/− WNT7A CM (72 hr, 100 nM sRECK-Fc) by Protein A agarose. Beads were incubated with 0.1, 1, or 10 μM sFZD4/sFZD8 protein (wedges) or glycine, pH 2.9 for 1 hr (eluate 1) and then with 1% SDS (eluate 2).
(E) WNT7A binding to RECK and FZD8 is mutually exclusive. HEK293 RECK−/− WNT7A cells were cultured in medium supplemented with indicated proteins (25 nM) for 72 hr. CM were subjected to sequential Ni-NTA agarose (1st) and Protein A agarose (2nd) pull-downs.
(F) RECK dose-dependently enhances FZD8:WNT7A complex formation. HEK293 RECK−/− WNT7A cells were cultured in medium supplemented with sFZD8-His and sRECK-Fc proteins as indicated for 72 hr. CM were subjected to Ni-NTA pull-down.
(G) Model of RECK/GPR124-regulated canonical WNT7 signaling. GPR124 ECD binding to RECK strongly enhances RECK/WNT7-induced canonical Wnt signaling by an extracellular mechanism that does not involve intrinsic GPR124 signal transduction or regulation of RECK:WNT7 complex formation. The RECK cystine knot motifs (blue) and cysteine-rich domain (purple) mediate binding of RECK:GPR124 to WNT7, stabilizing WNT7 in its active, monomeric, hydrophobic form. Free WNT7 rapidly converts into an inactive, aggregated, hydrophilic state. The GPR124:RECK complex acts as a stabilizing receptor for WNT7 increasing bioavailability of active, monomeric cell surface WNT7. GPR124:RECK-bound WNT7 is eventually transferred to FZD and LRP5/6 co-receptors by transient direct or indirect interactions. Alternatively, GPR124/RECK/WNT7 could form a stable multi-protein receptor complex with FZD/LRP5/6.
All western blots (WB) were performed under reducing conditions. All data are representative of at least two independent experiments with similar results. CM, conditioned medium; s, soluble ECD; Fc, IgG Fc fragment.
See also Figure S5.