Abstract
Thyroid-associated ophthalmopathy (TAO) is a complex disease process presumed to emerge from autoimmunity occurring in the thyroid gland, most frequently in Graves disease (GD). It is disfiguring and potentially blinding, culminating in orbital tissue remodeling and disruption of function of structures adjacent to the eye. There are currently no medical therapies proven capable of altering the clinical outcome of TAO in randomized, placebo-controlled multicenter trials. The orbital fibroblast represents the central target for immune reactivity. Recent identification of fibroblasts that putatively originate in the bone marrow as monocyte progenitors provides a plausible explanation for why antigens, the expressions of which were once considered restricted to the thyroid, are detected in the TAO orbit. These cells, known as fibrocytes, express relatively high levels of functional TSH receptor (TSHR) through which they can be activated by TSH and the GD-specific pathogenic antibodies that underpin thyroid overactivity. Fibrocytes also express insulin-like growth factor I receptor (IGF-IR) with which TSHR forms a physical and functional signaling complex. Notably, inhibition of IGF-IR activity results in the attenuation of signaling initiated at either receptor. Some studies suggest that IGF-IR-activating antibodies are generated in GD, whereas others refute this concept. These observations served as the rationale for implementing a recently completed therapeutic trial of teprotumumab, a monoclonal inhibitory antibody targeting IGF-IR in TAO. Results of that trial in active, moderate to severe disease revealed dramatic and rapid reductions in disease activity and severity. The targeting of IGF-IR with specific biologic agents may represent a paradigm shift in the therapy of TAO.
Essential Points
Thyroid-associated ophthalmopathy (TAO) is incompletely understood, and thus, existing treatments are nonspecific and suboptimal and fail to alter disease outcomes
The involvement of TSH receptor (TSHR) in TAO is not fully understood
Recently recognized monocyte progenitor cells, called fibrocytes, infiltrate the TAO orbit, express several “thyroid-specific” proteins, and respond to pathogenic antibodies in Graves disease
Insulin-like growth factor I receptor (IGF-IR) is overexpressed in several cell types in TAO, including fibrocytes and orbital fibroblasts (OFs), and forms a physical and functional complex with TSHR, and its activity is necessary for mediating components of TSHR downstream signaling
Inhibition of IGF-IR activity with specific monoclonal antibodies can attenuate the induction by TSH of specific gene expression in fibrocytes and OFs
An anti-IGF-IR antibody, teprotumumab, has recently been shown effective in reducing several manifestations of TAO, including those that were previously amenable only to surgical rehabilitation
Recent advances in our understanding of autoimmunity provide an ever-expanding context in which to view those diseases affecting the thyroid. Yet, the pathogenesis of Graves disease (GD), the most common thyroid autoimmune disease, remains incompletely understood. In particular, the relationship between manifestations occurring within the thyroid gland and those affecting connective tissues remains to be elucidated. Thyroid-associated ophthalmopathy (TAO) is a poorly managed component of GD for which there are no medical therapies with proven abilities to alter the outcome of disease. This unmet public health need results from the deficits in our insights concerning disease mechanisms. Recent identification of the insulin-like growth factor I receptor (IGF-IR) as a potential therapeutic target for TAO is now invigorating inquiry into potentially intersecting components of the IGF-I pathway and autoimmunity. This article attempts to describe the current landscape for TAO and how insinuation of IGF-IR into the growing list of therapeutic target candidates might improve the clinical care of this vexing condition.
Description of TAO
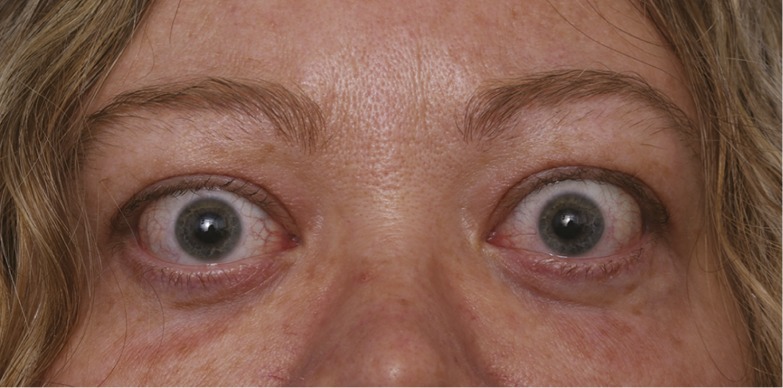
TAO is a disfiguring and potentially sight-threatening autoimmune disease most frequently found complicating GD (1). It also occurs in association with Hashimoto’s thyroiditis, but this is considerably less common. By virtue of its low incidence, TAO is considered to be an orphan disease. The soft tissues around the eye, including those within the bony orbit and upper face, become inflamed and undergo remodeling, leading to dysfunction of adjacent structures (Fig. 1). The eye itself is not primarily targeted by the disease but can be secondarily affected. A component of TAO results directly from the constraints imposed by the bony walls of the orbital space and the crowding of expanding soft tissues, potentially harming the globe and its vascular supply and innervation. Despite its description nearly two centuries ago, no medical treatment of TAO has been approved by the US Food and Drug Administration.
Figure 1.
Image of patient with TAO. [© 2019 Illustration Presentation ENDOCRINE SOCIETY].
A widely held view embraces the concept that the pathogenic underpinnings of thyroid glandular dysfunction (most commonly hyperthyroidism) and extrathyroidal manifestations of GD (of which TAO is the most important example) are very similar if not identical. Age and sex appear to exert important influences on the incidence and severity of TAO in the population of patients with GD (2, 3). Children with GD rarely manifest clinically important TAO, and when they do, the disease is usually not severe (4). The overall incidence of TAO appears to have declined in the recent past for unknown reasons (5, 6). Reduction in tobacco use is frequently cited as a major cause of this decline. Estimates of the yearly incidence of TAO in the United States are frequently based on a 20-year-old study conducted in Olmstead County, Minnesota. That study found ~16 cases per 100,000 in women and 2.9 per 100,000 in men (7). In another multicenter study, one-third of patients with TAO gave a positive family history of thyroid autoimmunity, and 12% reported consanguineous relatives with other autoimmune disease (8).
Presentation and clinical course of TAO
Clinically important TAO complicates ~40% of patients with GD (1, 9). A wide spectrum of disease presentation, severity, and clinical features can sometimes make arriving at a correct and timely diagnosis challenging. This is particularly true if the ocular manifestations of GD precede those of thyroid dysfunction, are unilateral, or exhibit marked asymmetry. When the cause of ocular manifestations is not clear cut, then orbital imaging with computed tomography or MRI is warranted. Imaging is also frequently helpful in assessing the distribution and progression of disease manifestations within the orbit and for assessing the integrity of the optic nerve. TAO is usually bilateral but can be markedly asymmetrical. In such cases, the distinction of TAO from other diagnoses, such as orbital tumors, pseudotumors, or orbital infections, becomes essential (10). The disease affects not only the orbital contents, including both the connective tissue fat depot and extraocular muscles, but can also alter the skin and subcutaneous tissues of the upper face. TAO follows a typical pattern of development and runs a course of activity that predictably is followed by stabilization. The initial, active phase usually lasts 1 to 3 years, as was described by Rundles curve (11). The duration of activity is highly variable and can last a few months or persist for many years. The active phase has, as its hallmark, progression of symptoms and signs, such as eyelid retraction, inflammatory changes, congestion, and edema of the periocular tissues, including the eyelids. Abnormalities in extraocular muscle motility can dominate disease presentation. Features of TAO can resemble those of ocular myasthenia gravis, a disease that can coexist with GD (12). The signs and symptoms vary greatly among cases. Dry eye and increased lacrimation are commonplace and are the consequence of poor eyelid coverage and diminished tear film quality (13). The integrity of the ocular surface can be violated, potentially threatening the health of the eye. When severe enough, it can lead to blindness. Proptosis develops in some but not all patients during the active phase of the disease (14). This anterior displacement of the globe results from increased volume of the soft tissues within the orbit. Either expansion of orbital fat or extraocular muscles can dominate the change in volume, but in the majority of patients developing proptosis, enlargement of both tissues contributes to the process. Proptosis can recede or worsen as disease activity runs its course and behaves unpredictably. Whereas proptosis can compromise the vitality of the optic nerve, volume expansion in the absence of forward globe displacement can also result in nerve compression and visual loss. Whatever its cause, compressive optic neuropathy represents an ophthalmic emergency necessitating urgent action. This remediation frequently involves surgical orbital decompression if high-dose intravenous glucocorticoid therapy fails to provide rapid improvement (15, 16). Another major source of morbidity in TAO results from diplopia, which if severe, requires remedial surgical correction. Fresnel prisms can offer rapid, if only partial, relief from debilitating diplopia. Severe diplopia is a major contributor to the diminished quality of life (QOL) associated with TAO.
Following cessation of disease activity, TAO typically lapses into the stable phase. In some cases, the signs of tissue congestion and inflammation lessen, and proptosis improves. In others, these persist and fail to change. The temporal boundaries between active and inactive TAO are frequently blurred, making the distinction between the two phases challenging. Because performance of most nonurgent surgical rehabilitation is reserved for the period following activity, the accurate judgment of when the stable disease phase has been reached carries important practical importance. Once stable, TAO only rarely becomes reactivated. When it does occur, reactivation can often be linked to an inciting event, such as ocular surgery or treatment of hyperthyroidism with radioactive iodine (RAI), followed by a poorly controlled thyroid function. Smokers are more likely to experience disease reactivation and are widely considered to experience more severe disease than do nonsmokers (17).
Another aspect of the clinical behavior exhibited in GD is the potential relationship between TAO and changes in thyroid gland activity. This is reflected by altered levels of thyroid hormones and TSH. Gland dysfunction develops in a majority of patients with GD, sometime in the course of the disease. Hypothyroidism may worsen the disease—effects that have been attributed to elevations in serum TSH. Similar worsening and reactivation following RAI ablation have been linked to increases in autoantibody levels, as well as poorly managed hypothyroidism, resulting from thyroid ablation (18). This possibility has led some experts to advocate very early assessment of thyroid function following successful gland ablation, thus allowing early correction of abnormal thyroid hormone levels (19). Whether alterations in thyroid function per se contribute to the development or worsening of TAO remains uncertain. Recent evidence from studies conducted in vitro suggests that the thyroid hormone analog, tetrathyroacetic acid—the levels of which are elevated in GD—may enhance the actions of both TSH and thyroid stimulating immunoglobulin (TSI) in orbital fibroblasts (OFs) (20). Those findings identify a potential mechanism through which thyroid status might directly influence the immunopathology occurring within the orbit.
Impact of treatments for hyperthyroidism on TAO
An area of considerable debate concerns the impact that choice of therapy for hyperthyroidism might exert on TAO (21). Although considered at certain institutions to be first-line therapy for hyperthyroidism, many investigators feel that RAI ablation should be avoided in patients with moderate to severe TAO. Acharya et al. (22) systematically reviewed the evidence that RAI administration carries a small risk for development and progression in TAO (23). Glucocorticoids can help mitigate the negative impact associated with RAI ablation on the behavior of TAO (24). Evidence that surgical thyroidectomy, independent of other variables, influences the course of TAO remains inconclusive (25, 26). Thus, decision as to the treatment modality best used in patients already manifesting TAO is based more on opinion than on scientific evidence.
Putative Genetic, Epigenetic, and Environmental (Acquired) Underlying Factors
Autoimmune thyroid diseases, such as GD, are generally considered to develop in individuals when genetic susceptibility intersects with acquired factors, the identities of which remain uncertain. Patients with GD carry strong, heritable components of risk, based, in part, on several large-scale genetic studies (27) and monozygotic twin studies (28, 29). The shared occurrence of TAO in both GD and Hashimoto’s thyroiditis should not be surprising, given the common susceptibility genes overarching the two diseases (30–32). Whereas several strong gene candidates identified include those also implicated in nonendocrine autoimmune diseases, others appear to be thyroid disease specific.
Considerable effort has been expended in identifying the genetic basis for GD. The number of susceptibility genes thus far recognized for GD has increased progressively, as results of additional genome-wide linkage analyses, candidate analyses, and family linkage studies have been reported (27–32). Many of the genes conveying predisposition for thyroid autoimmunity are located in the human leukocyte antigen region, such as human leukocyte antigen-DR3, and may determine disease behavior (33). Another strong candidate is TSH receptor (TSHR), where variants might allow a wider anatomic distribution of the autoantigen (34–37). Lower intrathymic expression of TSHR variants could result in less-efficient editing of antigen-specific autoreactive T cells, thus allowing their escape from central tolerance. Five GD-related polymorphisms have been identified in a 40-kb sequence of intron 1 of TSHR. The gene locus for TSHR, 14q, contains a neurexin gene, NRXN3, another candidate in GD (38). Neurexins are proteins that function as cell adhesion molecules (39). The cytotoxic T lymphocyte antigen 4 gene regulates T cell behavior (40) and is another strong candidate for GD and TAO (41), as are protein tyrosine phosphatase-22 (42) and CD40 (43). Polymorphisms of B lymphocyte activating factor have been found to be associated with thyroid autoimmunity (44). An association of GD with the melatonin receptor has also been reported (45). A number of studies have examined genes encoding several interleukins [recently meta analyzed by Wong et al. (46)], none of which identified polymorphisms strongly associated with TAO. Whichever genes underpin GD and TAO, these factors in aggregate appear to account for only ∼30% to 50% of disease risk. It has not yet been possible to identify unambiguously genetic contributions specific for TAO apart from those underlying GD. This has led to speculation that the genetic underpinnings of TAO and GD are identical. It would appear that most, if not all, of the heritable underpinnings of GD are relevant to TAO as well.
“Fibrocytes express autoantigens associated with several autoimmune diseases.”
The combination of factors, genetic and acquired, underlying susceptibility and development of GD remains uncertain (47). Epigenetic alterations associated with GD have been reviewed recently (48). A number of examples of gene silencing, histone modification, and alterations in DNA methylation patterns have been identified; however, these have, thus far, failed to inform an overall theme that can mechanistically link together the majority of cases with GD. To date, epigenetic signatures that are specific for GD and TAO remain unidentified.
Genetic and epigenetic alterations of the IGF-IR gene have focused, in large part, on those potentially associated with growth retardation, such as the Silver-Russell syndrome (49), longevity (50), polycystic ovary syndrome (51), dementia (52), and various cancers (53). An IGF-IR polymorphism (+3179G/A) may be associated with systemic lupus erythematosus (54). To our knowledge, the question of whether genetic alterations of IGF-IR or other components of the IGF-I pathway are associated with any form of thyroid autoimmunity, including GD and TAO, has not been explored in large-scale studies to date.
As with other autoimmune diseases, the identities of acquired, environmental factors triggering TAO remain uncertain. The potential relationship between infections, such as those involving Yersiniaenterocolitica, were recognized from studies demonstrating TSH and TSI binding sites in those bacteria (55, 56). Many patients that develop GD report acute life stresses, such as the loss of a close relative or recent incarceration. Cigarette smoke and antecedent infections appear to represent important acquired factors for the development of TAO. Tobacco smoke is frequently blamed for worsening of the disease (57). Selenium status, as an important determinant of TAO behavior, has been advocated by some studies (58) but not others (59). Dietary iodine content can profoundly influence thyroid abnormalities and the development of GD (60). Vitamin D deficiency is said to be prevalent in some cohorts, including one of Japanese women with GD (61). Polymorphism of the vitamin D receptor (62) has been found to be associated with GD in some groups of patients (62, 63) but not in others (64, 65). Likewise, variations of the vitamin D-binding protein gene have been reported (66).
Pathogenesis of TAO
Lack of rapid advancement toward a more complete understanding of TAO has directly impeded the development of specific therapies that target relevant disease-mediating pathways. To be certain, advancement in our understanding of TSHR structure, expression, and immunological characteristics has yielded important insights into the pathogenesis of GD and TAO. Whereas TSHR certainly plays a critical role in the hyperthyroidism of GD, the nature of its direct participation in TAO must still be clarified (Fig. 2). Much of the largely circumstantial evidence for involvement of TSHR and stimulatory anti-TSHR antibodies (TSI) in TAO derives from the finding that the receptor is expressed in orbital tissues, albeit at extremely low levels, compared with those found in thyroid epithelial cells (67, 68). Despite these low levels, TSH and TSI elicit responses in cultured OFs from patients with the disease (GD-OF). The concentrations of TSH and TSIs (frequently M22, a monoclonal stimulatory anti-TSHR antibody) (69) used in those studies, however, often have been well above those occurring under physiological conditions in vivo or in the context of disease (70–72).
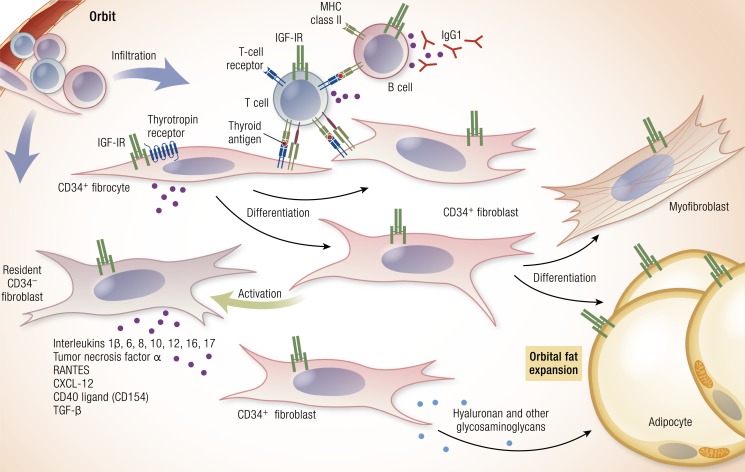
Figure 2.
Theoretical schematic of the pathogenesis of TAO. Infiltrating CD34+ fibrocytes emanating from the monocyte lineage in bone marrow enter from the circulation and set the stage for disease. They express and present autoantigens reminiscent of those found in the thyroid gland. Among these are low levels of TSHR, thyroglobulin, and other thyroid antigens. Fibrocytes present antigens to antigen-specific T cells, which in turn, endorse the production of IgG1 by B cells. These fibrocytes differentiate into CD34+ fibroblasts that can differentiate further into myofibroblasts and adipocytes, depending on the signals they receive. CD34+ fibroblasts encounter residential CD34− fibroblasts. When activated, these cells generate many proinflammatory and anti-inflammatory factors, including cytokines. Among these are IL-1β, -6, -8, -10, -12, and -16; TNF-α; the chemokine known as “regulated on activation, normal T expressed and secreted” (or RANTES), CXCL12, and CD40 ligand (CD40L; CD154). These cytokines can act locally on virtually all of the cellular inhabitants of the TAO orbit. Virtually all of the infiltrating and residential cell types display IGF-IR. Thus, therapies based on the inhibition of IGF-IR might target any and all of these cells. Cytokine-activated fibroblasts synthesize hyaluronan (HA) and other glycosaminoglycans, which expand orbital tissue volume and can lead to development of proptosis. In TAO, orbital fat can expand as a likely consequence of adipogenesis. [Reproduced with permission from Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016;375(16):1552–1565. Copyright Massachusetts Medical Society; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Central role for OFs
The findings of studies thus far conducted that examine the putative cellular targets in TAO suggest strongly that GD-OFs are selectively activated by the immune system (73). This population of cells, derived from the adipose fat depot (usually in the deep orbit), exhibits marked phenotypic heterogeneity with regard to its display of cellular markers and its behavior in culture (74). GD-OFs are particularly responsive to cytokines and other inflammatory mediators (75–78). Fundamental differences between GD-OFs and fibroblasts from healthy orbits have emerged (79). They have a capacity to engage in terminal differentiation, including undergoing adipogenesis (80).
Distinct GD-OF subsets have been identified, based on the array of surface and cytoplasmic determinants that these cells express. Initially, display of CD90 (Thy-1) was recognized as varying among populations of GD-OFs and was used to distinguish fibroblasts with differing capacities for gene expression, cytokine release, and terminal differentiation (81). In findings analogous to those from earlier studies conducted with lung fibroblasts, Thy-1+ GD-OFs were shown to differentiate into smooth muscle actin-α-expressing myofibroblasts when treated with TGF-β, actions mediated through the Smad pathway. In contrast, Thy-1− GD-OFs transition into triglyceride-accumulating adipocytes when treated in vitro with peroxisome proliferator-activated receptor γ (PPARγ) agonists (82). Each Thy-1 subset exhibits a characteristic profile of cytokine production. Both generate IL-6 when activated with IL-1β and CD40 ligand (CD40L; aka CD154) (82). In contrast, Thy-1+ cells generate higher levels of prostaglandin E2 (PGE2) as a consequence of the induction of prostaglandin endoperoxide H synthase 2, the inflammatory cyclooxygenase, when they are exposed to proinflammatory cytokines, such as leukoregulin (75), IL-1β (83), and CD40L (84). Induction of prostaglandin endoperoxide H synthase 2 in GD-OFs is coordinated with that of the terminal glutathione-dependent PGE2 synthase (83). Accumulation of PGE2 in the TAO orbit can alter the immunological environment of those tissues, a consequence of changes in T helper (Th) cell skew and enhanced immunoglobulin production by B cells (85, 86). IL-8 can be induced preferentially, and the induction by interferon γ of major histocompatibility complex class II (MHC-II) is considerably greater in Thy-1− fibroblasts. In contrast to the heterogeneity exhibited by GD-OFs from orbital fat, those cells from the extraocular muscles, namely perimyseal fibroblasts, uniformly express Thy-1+ (74). These cells fail to differentiate into adipocytes when exposed to PPARγ ligands. They appear to be considerably more homogeneous than those from orbital fat.
A number of other cell markers expressed by GD-OFs have been identified, and these also allow demarcation of functionally discrete cells. As a group, they express several proteins associated with stem cells, including the intermediate filament, nestin; CD146; and platelet-derived growth factor (PDGF) receptor (87). Many of these characteristic cell markers distinguish GD-OFs from “garden variety” OFs coming from healthy individuals with regard to several phenotypic attributes. GD-OFs appear to be more susceptible to the cell shape-deforming effects of agents that enhance the production and accumulation of intracellular cAMP (77). They respond in a characteristic pattern to proinflammatory cytokines, such as IL-1β, leukoregulin, CD40L, PDGF, and IL-17A (84, 88–90). Among the target genes exhibiting remarkably robust inductions, in addition to enzymes in the PGE2 biogenic pathway, are numerous cytokines and enzymes belonging to the hyaluronan (HA) biosynthetic pathway, such as the HA synthases (91) and uridine diphosphate glucose dehydrogenase (92, 93).
Evidence that CD34+ fibrocytes infiltrate the TAO orbit
Fibrocytes have been implicated in several diseases involving chronic inflammation and fibrosis that frequently culminate in the marked disruption of normal tissue architecture, function, and pliability (94). They may be involved in the pathogenesis of rheumatoid arthritis (RA) (95), diabetes mellitus (96), and scleroderma (97). Potentially relevant to TAO, identification among GD-OFs of a fibroblast subset exhibiting the CD34+CXCR4+collagen I+ phenotype prompted our consideration of whether they constitute a discrete population infiltrating the orbit (98) (Fig. 3). Cells within the GD-OF population with this array of markers (referred to hereafter as CD34+ OF) meet the widely accepted criteria for fibrocytes, bone marrow-derived progenitor cells of the monocyte lineage (99). These cells play important roles in tissue remodeling, such as those involved in wound repair. Fibrocytes generate collagen and other extracellular matrix molecules, and their activities can result in fibrosis. Fibrocytes cultivated from circulating peripheral blood mononuclear cells (PBMCs) are capable of antigen presentation, expressing high levels of constitutive MHC-II and several costimulatory molecules (100, 101). They can prime T cells and are dependent on contact with these lymphocytes for their own differentiation (102).
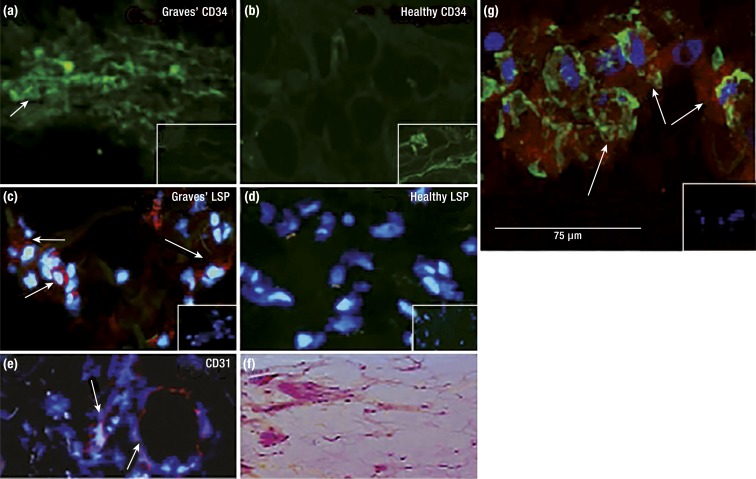
Figure 3.
CD34+ fibrocytes infiltrate the orbit in TAO. OFs derived from patients with TAO comprise discrete subsets of cells on the basis of CD34 display. CD34+lymphocyte-specific protein (LSP) 1+TSHR+ fibrocytes are present in TAO orbital tissue but not in healthy tissues. (a) CD34 expression (arrows) in TAO-derived tissue (inset, negative control). (b) Undetectable CD34 in healthy tissue (inset, positive control). (c) LSP-1 expression in TAO tissue [red; arrows (inset, negative control)]. (d) Undetectable LSP-1 in healthy tissue (inset, negative control). (e) CD31 expression in TAO tissue is limited to vascular endothelium (red; arrows). (f) Hematoxylin and eosin-stained, consecutive thin sections of the same orbital tissue. (g) Fibrocytes present in TAO orbital tissue coexpress CD34 and TSHR. Arrows denote fibrocytes. [Reproduced with permission from Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–438; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
In the context of TAO, fibrocytes become substantially more abundant in the circulation of patients with GD (98). Recent evidence indicates that fibrocytes express autoantigens associated with several autoimmune diseases (103). They also express multiple “thyroid-specific” proteins, including TSHR, thyroglobulin, sodium-iodide symporter, and thyroperoxidase (103–105) (Fig. 4). Expression of these proteins appears to depend on the autoimmune regulator protein (AIRE), as knocking down its levels substantially reduces their abundance. Moreover, patients with autoimmune polyglandular syndrome-1 and harboring a loss-of-function mutation of the AIRE gene express considerably reduced levels of these same thyroid proteins (104). CD34+ OF appear to represent fibrocytes from the circulation that have infiltrated the orbit and have become morphologically indistinguishable from residential OFs. They copopulate the tissues with CD34− OF, cells that in the healthy orbit account for the entire population of OFs. Although varying among cases, it appears that approximately one-half of cells comprising GD-OF strains cultivated in vitro are CD34+ OF (98). Comparisons between CD34+ OF and CD34− OF disclose important differences in phenotype. These distinctions, however, are dramatically enhanced when the mixed populations of GD-OFs are subjected to cell sorting into pure CD34+ OF and CD34− OF subsets (104, 105). Several genes become substantially upregulated in CD34+ OF when they are removed from the molecular context created by CD34− OF. It appears that CD34− OF express and release a soluble factor(s) that represses the expression of specific genes in CD34+ OF. These genes are expressed at considerably higher levels in fibrocytes from the peripheral circulation than by CD34+ OF. Removal of CD34+ OF from the cultural context of CD34− OF partially restores AIRE and thyroid gene expression to the levels more closely resembling those found in fibrocytes cultured from the peripheral circulation (105). Our laboratory group has very recently identified the inhibitory factor expressed by CD34− OF as the axon-repellent protein, Slit2 (106). In GD-OFs, Slit2 is highly inducible by TSH and M22.
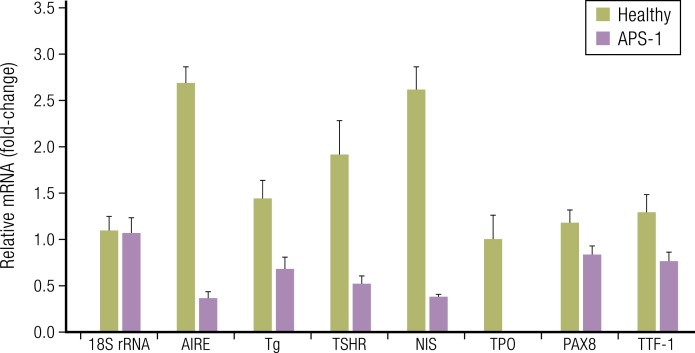
Figure 4.
Fibrocytes express several proteins thought to be restricted to the thyroid. Expression of the proteins appears to depend on autoimmune regulator protein (AIRE). Fibrocytes collected from an individual with autoimmune polyendocrine syndrome type 1 (APS-1) express lower levels of AIRE, TSHR, thyroglobulin (Tg), sodium iodide symporter (NIS), thyroid peroxidase (TPO), pair-boxed 8 (PAX8), and thyroid transcription factor-1 (TTF-1) than do those from an unaffected, first-degree relative. Results from real-time PCR of the targets are indicated. [Reproduced with permission from Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of TSHR, thyroglobulin, sodium iodide symporter, and thyroteroxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99(7):E1236–E1244; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Orbit-infiltrating mononuclear cells as drivers of TAO
The role of adaptive immunity in TAO is firmly established and is likely to be either very similar or identical to those processes occurring within the thyroid in GD. Several studies have examined the characteristics of lymphocytes that are variably present in the orbit during active TAO (107, 108). Among them are both T cells with effector function and those with regulatory phenotypes (109). It remains uncertain what antigen might be specifically attracting T cells to the orbit in active disease and which cell types might dominantly participate in antigen presentation once disease-relevant T cells have infiltrated the tissue. Orbit-infiltrating B cells have been partially characterized (110). Whereas intraorbital dendritic cells have yet to be identified, CD34+ OF, putative derivatives of monocyte-lineage fibrocytes, could play important roles in antigen presentation to T cells in TAO by virtue of their constitutive expression of MHC-II (98, 100). Any generation of IgG1 within the orbit would require T cell endorsement. The CD40/CD40L bridge allows crosstalk between T cells and GD-OFs (84, 111). It may account for at least a component of the characteristic gene expression and cytokine generation found in TAO (84, 112, 113). Th17-polarized T cells and IL-17A have been implicated in the disease (88, 114). This cytokine can induce a characteristic set of GD-OF genes associated with TAO, including regulated on activation normal T cell expressed and secreted (RANTES). Mast cells have also been found in orbital-connective tissues (115), and the interactions between these cells and GD-OFs appear to carry functional consequences (116, 117).
Tissue remodeling in the orbit characteristic of TAO
A cardinal feature of the changes appearing in connective tissues within the TAO orbit is the accumulation of glycosaminoglycans, of which HA has been identified as the predominant type (118, 119). By virtue of its unique rheological properties, HA possesses the capacity to bind enormous amounts of water. In the hydrated state, it occupies an extremely large volume. This, in turn, causes the tissues within the orbit to expand, creating the characteristic anterior propulsion of the globe, which frequently results in proptosis (14). A major source of HA production appears to be the GD-OFs and is driven by cytokines, such as IL-1β, leukoregulin, CD40L, GD-IgG, and growth factors (78, 120). Both IGF-I and GD-IgG induce HA accumulation in GD-OFs. These actions appear to be peculiar to cells from patients with TAO and can be attenuated with 1H7, a monoclonal murine anti-IGF-IR blocking antibody (120). This accumulation appears to be caused by increases in rates of macromolecular synthesis (91), as degradation of HA in human fibroblasts is nil (121). Later in the course of the disease, fibrosis can dominate the tissue changes and can directly lead to deficits in extraocular muscle motility and result in diplopia (14). Fibrosis of the extraocular muscles can dominate the clinical picture of severe, stable TAO (14). This can account for substantial disease-related morbidity.
Involvement of TSHR and TSI in TAO
TSHR, initially cloned by Parmentier et al. (122), plays multiple roles, both directly and indirectly, in regulating fat metabolism and energy expenditure. Its expression increases as mesenchymal stem cells differentiate into fat (123). Furthermore, TSHR activation increases adipogenesis in abdominal wall preadipocytes. A relationship between TSHR and leptin expression has been described. Leptin mRNA levels were found to be elevated in fat from patients with GD (123). In hypothyroid mice, TSH acts as a lipolytic factor (124). Acute administration of recombinant human (rh)TSH to hypothyroid human beings increases serum leptin levels that are proportional to body fat mass (125). In contrast, TSH reduces leptin expression in rat adipocytes in vitro (126).
Multiple thyroid autoantibodies can be detected in patients with GD. These include antibodies against thyroglobulin and thyroperoxidase that are considered to be nonpathogenic and not disease specific. On the other hand, they are peculiar to thyroid autoimmunity. In contrast, loss of immune tolerance to TSHR and the generation of TSI are central, diseases-specific events in the development of GD (1, 127). Apparently, the TSHR epitope to which these antibodies target determines the receptor signaling that they elicit (128). Unlike events occurring within the thyroid, the role of TSHR and TSI in the pathogenesis of TAO is considerably less well defined. Evidence that these autoantibodies and TSHR are involved, directly or indirectly, is sizable but largely circumstantial. TSHR participation in the development of orbital pathology remains a very attractive concept, one that is congruent with the orbit and thyroid gland sharing a common autoantigen(s). Among the findings either supporting or challenging this involvement include the following: (i) TSIs can be detected in the vast majority of patients developing TAO; however, these antibodies are also detectable in most individuals with GD and hyperthyroidism but in whom clinically meaningful TAO fails to develop. Importantly, infrequent patients, some with severe TAO, have presented with undetectable TSI (129). (ii) Recent findings suggest a relationship, perhaps predictive in nature, between the levels of TSIs and the clinical behavior of TAO (130). (iii) TSHR can be detected in orbital-connective tissues and the fibroblasts derived from those tissues (67, 70). (iv) TSH and TSIs can induce several proteins in GD-OFs and CD34+ fibrocytes in vitro (71, 72, 98). It should be noted, however, that TSHR expression and responses to TSH have also been demonstrated widely in connective tissue depots and derivative fibroblasts (68, 98, 131). Many of those tissues rarely, if ever, manifest GD. Thus, the relationship among TSHR, TSI, and TAO remains incompletely understood.
Animal models of TAO
A major barrier to more complete understanding of TAO has been the lack of preclinical models. Substantial effort has been directed in the development of rodent models of GD and importantly, those that also include orbital pathology resembling TAO. Models reported more recently appear to have achieved partial success. The earliest attempts at developing an autoimmune mouse model include those involving immunodeficient mice (132). A search for the conditions that would yield optimal anti-TSHR antibody generation following various immunization strategies was undertaken (133). Outbred mice were examined after genetic immunization with TSHR cDNA (134). Results from that study demonstrated a strong sex bias toward development of hyperthyroidism in female mice. The animals exhibited periocular edema, deposition of uncharacterized extracellular material, and mononuclear cell infiltration of the extraocular muscles. In contrast, many of the models successfully recapitulating antibody-mediated hyperthyroidism have failed to exhibit detectable ocular manifestations (135). Evidence has been generated supporting the importance of Th1 responses in these models of hyperthyroidism (136). It would appear that the shed extracellular A subunit of TSHR is considerably more immunogenic than the intact receptor in promoting both induction and amplification of the immune response in mouse models of GD (137). In spite of a more severe hyperthyroid phenotype, the authors failed to detect differences in anti-TSHR antibody generation, regardless of the structural form of TSHR. A mouse model in which TSIs from a patient with GD were expressed by B cells as a transgene resulted in persistent hyperthyroidism (138). An apparent advance in the generation of the hyperthyroid phenotype occurred when animals were immunized with TSHR DNA in a protocol that includes electroporation into targeted muscle as part of the delivery process (139). This approach resulted in a more durable immune response. With the use of a similar protocol, another study produced a majority of animals that became hyperthyroid and generated anti-TSHR antibodies (140). These animals also manifested orbital fibrosis. Unexpectedly, animals immunized with TSHR also generated anti-IGF-IR antibodies. A subsequent report from this same group revealed that identical immunizations could instead result in hypothyroidism rather than hyperthyroidism and could be accompanied by mononuclear cell infiltration of the orbit and enlargement of the extraocular muscles (141). A follow-up study was conducted by the same investigators but working at two distant institutions. With the use of the same experimental techniques, they described animals that developed hyperthyroidism in one center but the absence of this phenotype in the other geographic location. These disparate results led the authors to conclude that the determinants of thyroid function in their immunized animals were random (142). They did not offer convincing evidence for this supposition.
Thus, models for GD and TAO have improved materially in recent years. Several aspects of the animal phenotypes generated experimentally resemble those of human disease. However, their current limitations must also be acknowledged. Significant variability emerged in the animals generated using identical treatment protocols. Furthermore, notable differences persist between these animals and the human disease. This makes uncertain the value of their interrogation as a means for defining the underlying mechanisms of human disease. Considerable general debate exists concerning the suitability of animal models as surrogates for human disease (143, 144). The immune systems and anatomical structures of the respective orbits differ in important respects. In any event, some of these improved animal models should allow first approximation of disease response to therapy and as such, may be useful in proof-of-principle studies that could facilitate clinical trials.
Overview of IGF-IR Biology
IGF-IR biology
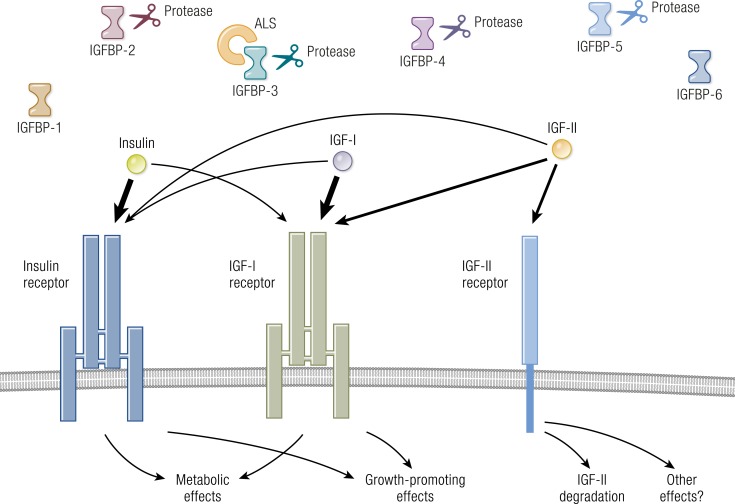
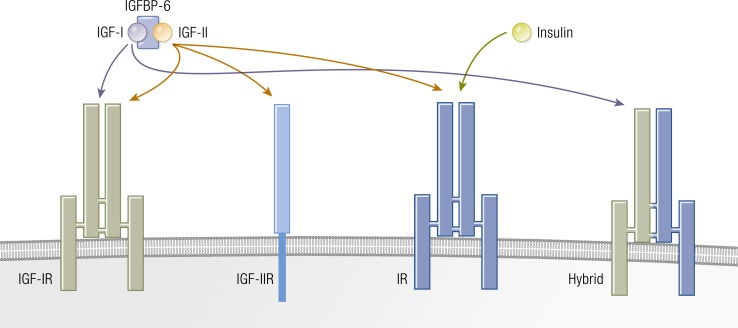
The IGF pathway comprises a complex network of proteins that regulate diverse biological functions through endocrine, paracrine, and autocrine mechanisms (Fig. 5). This pathway includes two growth factors (IGF-I and IGF-II), six IGF-binding proteins (IGFBP-1 through IGFBP-6), nine IGFBP-related proteins, and two cell-surface receptors (IGF-IR and IGF-IIR/mannose-6-phosphate receptor) (145–147). IGF-IR is a member of the insulin receptor (IR) family. IGF-IR and IR may have evolved through gene duplication of a common precursor receptor molecule (148). Consequently, the structures of the two receptors are homologous. Like many other tyrosine kinases, IGF-IR and IR are displayed on the cell surface (149) (Fig. 6). The gene encoding human IGF-IR is located on chromosome 15 and is expressed ubiquitously by many cell types (150). Its heterotetrameric protein structure includes extracellular ligand-binding domains located in two α subunits and two β subunits in which the kinase domains reside (150). These subunits are linked by two disulfide bonds. The β subunits include extracellular, transmembrane, and intracellular domains. A consensus ATP-binding sequence and multiple potential tyrosine phosphorylation sites are located in the β subunits (145). Unliganded IGF-IR is maintained in an inhibited state, where the transmembrane and intracellular domains are held apart (151). That state, where the ligand binding sites are unoccupied, might generate signals involved in the expression of imprinted genes and microRNAs (152). A ligand-dependent conformational change appears to induce IGF-IR activity. When IGF-I or another agonist ligand binds to the extracellular domain, a closer proximity of regions within the transmembrane domain occurs, resulting in autophosphorylation within the β subunit (151). Phosphorylation of three tyrosine residues (Tyr1131, Tyr1135, and Tyr 1136) plays a major role in the activation of the receptor (153). IGF-I and IGF-II bind IGF-IR with high affinity, whereas the binding of insulin occurs with considerably lower avidity (154). IGF-IR and the two IRs (IR-A and IR-B) can form hybrid receptors that are preferentially activated by IGF-I (145, 155). This prioritization results from the ability of IGF-I to activate monomeric IGF-IR, whereas only dimerized IR responds to insulin (156). The formation of hybrid receptors appears to be a stochastic event. At least 6 different IGF-IR/IR combinations are possible (149). IGF-IR can heterodimerize with either of two IR splice variants, as well as with members of other receptor families, such as that for epidermal growth factor (157). This apparent promiscuity in theory substantially enlarges the repertoire of signaling events which it might initiate.
Figure 5.
Overview of the IGF-I pathway. IGF-I, IGF-II, insulin, IGF-binding proteins (IGFBPs), IGF-IR, IGF-IIR, and insulin receptor (IR) form a complex pathway. IGF-IR predominantly regulates growth, whereas the IR dominates regulation of metabolism. IGF-IIR (mannose-6-phosphate receptor) regulates IGF-II degradation. IGF-I and IGF-II action is modulated by six IGFBPs. ALS, acid-labile subunit. [Adapted with permission from Lowe WL Jr. Insulin-like growth factors. Sci Med. 1996;3(2):62–71; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Figure 6.
IGF-IR, IGF-IIR, and IR are displayed on the cell surface. IGF-IR and IGF-IR/IR hybrid proteins are preferentially activated by IGF-I rather than insulin. [Adapted with permission from Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13(1):16–24; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
IGF-IR is expressed by virtually all human tissues and cell types (146, 158). Its surface density represents an important determinant of the magnitude of responses to IGF-I and the signaling pattern it provokes (159). Receptor abundance is principally determined by levels of IGF-IR gene expression, which in turn, results from a complex interplay of that gene with multiple transcription factors (159). Factors positively regulating IGF-IR expression include PDGF; fibroblast growth factor; estrogens; oncogenes, such as src, SV40, and c-myc; and the transcription factor, Sp1. In contrast, IGF-I and IGF-II, insulin, estrogen antagonists, WT1, and p53 can downregulate its levels (160). IGF-I lowers the IGF-IR surface display by enhancing translocation of the receptor protein to an intracellular pool (161). IGF-IR density is also regulated by GH and 3,5,3′-triiodothyronine (T3) (162). The IGF-I pathway appears to mediate many effects of PDGF and fibroblast growth factor (163, 164). Functional IGF-IR is required for several actions of PDGF and epidermal growth factor, supporting the interdependent signal transduction pathways mediating the effects of these growth factors (165–167).
Diverse functions of the IGF-I pathway
IGF-I was discovered by Salmon and Daughaday in 1957 (168) when they identified in serum the sulfation factor, the activity of which could be induced by GH. This factor stimulates incorporation of radioactive sulfate into cartilage glycosaminoglycans (168). Soon thereafter, it was found to enhance DNA synthesis (169, 170), proline incorporation into hydroxyproline (171), and RNA synthesis (169, 172). Sulfation factor was subsequently referred to as somatomedin (172), the activities of which could be attributed entirely to IGF-I and IGF-II (173, 174). Actions of both IGF-I and IGF-II are modulated by six IGFBPs and IGFBP proteases (146) and are mediated through their binding to IGF-IR (146, 175). IGF-IR is expressed ubiquitously, accounting for the widespread actions of these growth factors. IGFs regulate growth, development, and malignant cell transformation (145, 176). Activated IGF-IR results in increased cell size and confers resistance to apoptosis (177). In addition to promoting cell growth, IGF-IR activation may result in cell differentiation (177). For instance, under certain conditions, differentiation of myoblasts, osteoblasts, and adipocytes can be provoked by either IGF-I or IGF-II (178). IGF-I mediates the anabolic effects of circulating GH (175). It stimulates bone formation, protein synthesis, glucose uptake in muscle, neuronal survival, and myelin synthesis (175).
IGF-I and IGF-II play important roles in adipogenesis, the process through which adipocyte numbers can increase (179). Levels of locally expressed IGF-I in fat are comparable with those in liver. Moreover, IGF-I mRNA increases following cell differentiation, whereas IGF-IR transcript decreases (180, 181). Virtually all IGF-IR molecules displayed by mature adipocytes exist as IGF-IR/IR hybrids (182). Autophosphorylated homodimeric IGF-IR cannot be detected in mature adipocytes differentiated from 3T3 cells; rather, phosphorylation is limited to IGF-IR/IR hybrid receptors (182). The physiological significance of this divergent behavior between protein complexes has not been clarified. It does allow for substantial post-translational “fine tuning” of both IGF-I and insulin actions on target cells.
Growth factors, such as IGF-I, play important roles in the regulation of proliferation within large populations, but their relationships to rapid signaling events occurring within individual cells are complex. A recent study disclosed that acute cellular responses to IGF-I, such as the activation of Akt signaling in a population of fibroblasts, do not predict the proliferation patterns of individual cells (183). IGF-I actions in fibroblasts can be influenced by IGFBPs and their fragments (184). Because of its importance in regulating connective tissue metabolism throughout the body, it is likely that IGF-I influences tissues within the orbit in the context of TAO. It enhances proliferation and lipid accumulation in GD-OFs (185). These effects are mediated, at least in part, through induction of adiponectin, leptin, PPARγ, adipocyte fatty acid-binding protein, and fatty acid synthase. IGF-I enhances HA accumulation in GD-OFs but not in those cells from healthy donors (120).
IGF-IR, like IR, appears to play important roles in the unliganded state. Expression of both maternally and paternally derived, imprinted genes and microRNAs in mouse embryonic fibroblasts is apparently dependent on these receptors when their ligand binding sites are unoccupied (152). The absence of these receptors results in altered DNA methylation patterns and may be mediated through as-yet unidentified noncanonical mechanisms.
Meaningful determination of IGF-I levels in serum and other biological fluids/compartments presents substantial challenges. For instance, a major problem in the measurement of IGF-I in blood and other biological fluids is the potential interference caused by IGFBPs. Their presence may result in spurious estimates of growth factor concentrations. Therefore, these proteins must be extracted before assay performance (186). However, the removal of IGFBPs can also eliminate critical components of the molecular context in which IGF-I activity occurs by altering the modulating influences exerted by these proteins. The affinities exhibited for IGF-I and IGF-II by the six mammalian IGFBPs thus far identified are higher than those of IGF-IR (187). Thus, when IGF-I is bound to these IGFBPs, it becomes unavailable for binding and activating IGF-IR. Therefore, IGFBPs determine IGF-I bioavailability. IGFBP cleavage by specific proteases results in reduced affinities for IGF-I and the consequential increase in bioavailable IGF-I. Most extraction techniques are imprecise and can result in variable levels of residual IGFBPs and IGFBP fragments, yielding situations that render estimates of IGF-I concentrations potentially inaccurate (188). In addition, IGF-I immunoassays poorly reflect the stimulatory and inhibitory actions of circulating antibodies directed against IGF-IR (189). Thus, the importance of the selection of the proper assay method for the particular set of questions to be investigated cannot be overstated.
IGF-IR signaling
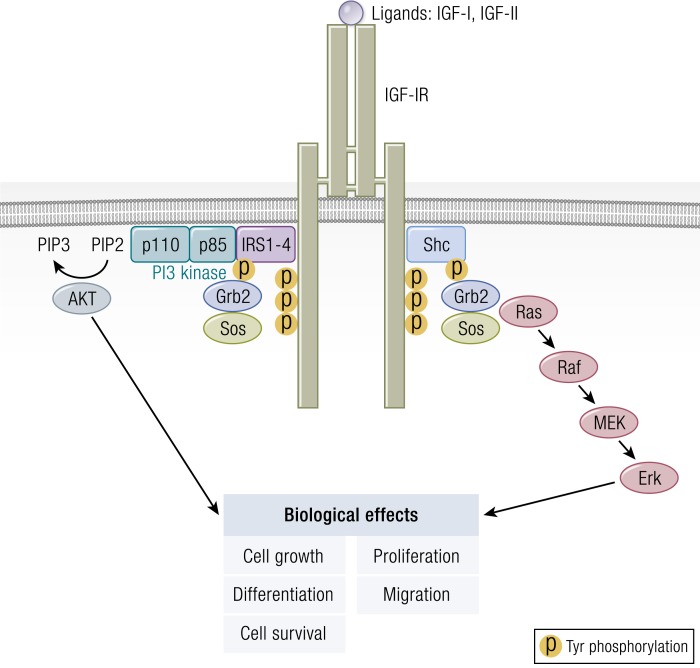
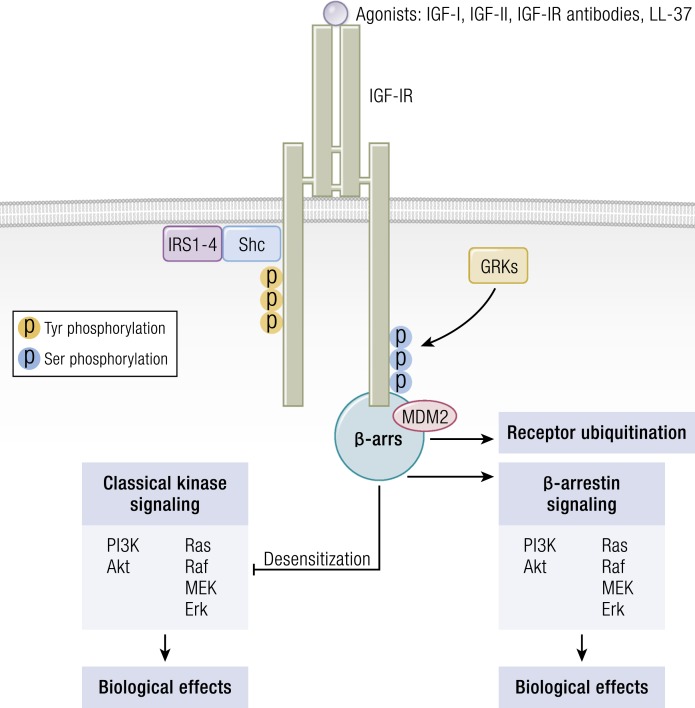
The classical model of signaling through IGF-IR centers on ligand-initiated kinase activation, culminating in adaptor molecule recruitment and binding (Fig. 7). This, in turn, results in downstream canonical MAPK/Ras-Raf-Erk and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) phosphorylation (190, 191). Recruitment of IR substrate (IRS), growth factor receptor-bound protein 2, and src homology domain-containing protein is critical to early postreceptor signaling events (191). MAPK mediates the actions of many ligands to cell-surface receptors, including those that alter cell proliferation, transformation, survival/death, and differentiation (192). IRS-I serves as the major substrate of IGF-IR and IR (193, 194). Phosphorylated IRS-1 binds to the p85 subunit of PI3K, leading to its activation (158). PI3K, in turn, generates inositol triphosphate and promotes activation of protein tyrosine kinase-B/AKT, mTOR, phosphoprotein 70 ribosomal protein S6 kinase (p70S6k), and glycogen synthase kinase 3-β (195). IGF-IR activity, like that of other receptor tyrosine kinases (RTKs), has been generally viewed as binary, either stimulating canonical PI3K-AKT and ERK pathways or existing in an inactive state (196). Initially, ubiquitin-mediated receptor downregulation and degradation were thought to result from agonist ligand/receptor interactions and thus, were considered inseparable from kinase activation (196). However, recent insights have proven this concept to be incorrect. Rather, IGF-IR “borrows” components of classical G-protein-coupled receptor (GPCR) signaling, including β-arrestins and G-protein-related kinases, as does IR. IGF-IR is now considered a functional RTK/GPCR hybrid that integrates canonical tyrosine kinase signaling with that of GPCRs (196–198) (Fig. 8). By virtue of its involvement in the functional RTK/GPCR hybrid, IGF-IR can engage in biased signaling, initially associated with classical GPCRs (199, 200). In biased signaling, either ligand or receptor is biased toward a specific signaling pathway (197). Interestingly, besides the blocking of IGF-IR, antibodies directed against that receptor can induce receptor internalization and simultaneously activate IGF-IR/β-arrestin-1-mediated ERK signaling, thus functioning as an IGF-IR/β-arrestin-1 agonist (201).
Figure 7.
Classical model of signaling through the IGF-IR. This results in downstream canonical (MAPK)/Ras-Raf-Erk and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) phosphorylation. IRS, IR substrate; MEK, MAPK kinase; P, phosphorylation. [Adapted from Worrall C, Nedelcu D, Serly J, Suleymanova N, Oprea I, Girnita A, Girnita L. Novel mechanisms of regulation of IGF-1R action: functional and therapeutic implications. Pediatr Endocrinol Rev. 2013;10(4):473–484; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Figure 8.
The IGF-IR is now considered a functional RTK/GPCR hybrid, integrating canonical tyrosine kinase and GPCR signaling. Ligand binding activates classical kinase-dependent signaling pathways, as well as β-arrestin recruitment to GPCR kinase-dependent, phosphorylated serine residues within the C-tail of IGF-IR. β-Arrestin (β-ARR1) induces kinase desensitization and receptor ubiquitination while provoking kinase-independent signaling through the MAPK pathway. [Adapted from Worrall C, Nedelcu D, Serly J, Suleymanova N, Oprea I, Girnita A, Girnita L. Novel mechanisms of regulation of IGF-1R action: functional and therapeutic implications. Pediatr Endocrinol Rev. 2013;10(4):473–484; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Important aspects of IGF-IR signaling have been revealed, with the identification of LL-37 as a partial agonist for the receptor (202). It represents the cleaved form of the only cathelicidin family member expressed in humans. LL-37 is an antibacterial peptide exhibiting agonistic activity toward IGF-IR by selectively activating ERK without affecting PI3K-AKT activity (202). ERK activation, provoked by LL-37, enhances cell migration and invasion in an IGF-IR/β-arrestin-1-dependent manner without affecting cell proliferation (202). Of potential relevance to TAO, LL-37 has already been implicated in several autoimmune diseases, including the periodontal disease associated with RA (203). Its levels are elevated in psoriatic skin (204), and it enhances survival of externalized DNA in systemic lupus erythematosus (205). Thus, the apparent role of IGF-IR signaling in TAO makes exploration of any potential involvement of LL-37 in the disease particularly intriguing.
Ligand-mediated endocytosis of many cell membrane-bound RTKs appears critical to their abilities to fine tune signaling (206). IGF-IR is monoubiquitinated at multiple sites, a process regulated by several E3 ubiquitin ligases and de-ubiquitinases (207). Ubiquitination induces receptor internalization and degradation, enhances signaling, and facilitates receptor trafficking (207). Signaling molecules downstream of IGF-IR are also ubiquitinated, thereby controlling their degradation and availability to form protein complexes. Thus, ubiquitination determines both receptor turnover and signaling efficiency (207). Its regulation serves as a broad governor of signaling initiated at membrane-spanning RTKs.
IGF-IR functions at the cell membrane but can translocate to the nucleus, where it may alter gene transcription (208, 209). Both IGF-I and GD-IgG can induce nuclear IGF-IR accumulation in GD-OFs, suggesting a potential role in TAO (210). Those actions are absent in OFs from healthy donors. Several factors besides classical receptor agonists appear capable of provoking this noncanonical behavior. Transthyretin can induce IGF-IR nuclear translocation (211). It upregulates IGF-IR transcription in NIH3T3 cells and in cultured neurons. This relationship represents a heretofore-unrecognized link between the thyroid hormone and IGF-I pathways, as thyroxine, like retinol, occupies a binding site on transthyretin (212). The biological significance of nuclear translocation, however, remains incompletely understood (208).
IGF-IR and immune function
IGF-I exerts regulatory actions on immune function and is expressed by professional immune cells and bone marrow stromal cells (213) (Fig. 9). These actions influence diverse pathways in immune cells through endocrine, paracrine, autocrine, and perhaps intracrine loops, thereby potentially impacting the pathogenesis of autoimmune diseases (214). Evidence supports its role in regulating both innate and adaptive immunity (215). IGF-I administration to mice following lethal irradiation and bone marrow grafting restored both B cell and T cell compartments (216). It resulted in substantial B cell expansion and a more modest increase in T cells (216, 217). Mature B cell and plasmacyte proliferation and antibody responses are enhanced by IGF-I (218).
Figure 9.
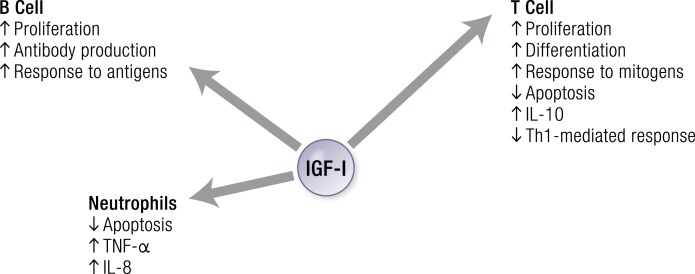
IGF-I exerts regulatory actions on immune function by stimulating B cells, T cells, and neutrophils. [© 2019 Illustration Presentation ENDOCRINE SOCIETY].
Many constituents of the PBMC surface display IGF-IR (219–221), including CD4+ and CD8+ T cells, B cells, monocytes, natural killer cells, and thymocytes (220, 222–227). Differences in receptor number/density and affinity were found among these cells (220, 226). For instance, monocytes and CD4+ Th cells exhibit higher levels of IGF-IR expression than do CD8+ cytotoxic/suppressor T cells (228). Human T cells fail to enter the S-phase following knockdown of IGF-IR, suggesting that the receptor protein is required for myeloid cell proliferation (229). T cell-expressed IGF-IR is functional and mediates effects on proliferation (230, 231) and chemotaxis (230). The half-maximal response to IGF-I for T cell proliferation is 0.12 nM (230) with binding maximum in CD4+ cells that is substantially greater than that in CD8+ T cells (232). IGF-IR density on T cells increases following mitogen activation in vitro (232). IGF-I enhances differentiation of thymic T cell progenitors (233). It promotes bone marrow pro-B lymphocyte differentiation during immunoglobulin gene rearrangement (234). Immunoglobulin production is stimulated by IGF-I in vitro and in vivo, as is class switching in plasma cells (235). IGF-I potentiates IL-7-driven expansion of pro-B-cells (236) in collaboration with c-kit ligand (237). In adult mice, it increases lymphocyte number and activity (217). Following IGF-I exposure, T and B cells isolated from spleen and lymph nodes are more responsive to mitogens and antigen stimulation, respectively (217). IGF-I increases natural killer cell cytotoxicity in vivo (220).
Human macrophages and granulocytes also display surface IGF-IR (228, 238). In neutrophils, IGF-I retards spontaneous apoptosis (228). It stimulates expression of cytokines and chemokines, such as TNF-α and IL-8 (239). IGF-I also promotes IL-2 synthesis by activated T cells and thus, behaves as a proinflammatory factor (240). In contrast, IGF-I can exert anti-inflammatory actions through the stimulation of IL-10 and inhibition of Th1-mediated cellular immune responses in activated T cells (217, 241).
A disproportionately large fraction of peripheral T cells expresses IGF-IR in GD, a consequence of CD45RO+ memory cell expansion. This expansion results in resistance to Fas-mediated apoptosis and enhanced proliferation (242). B cells in blood, orbit, and bone marrow are over-represented by those lymphocytes expressing IGF-IR (243). Receptor display on B cells is associated with enhanced survival and antibody production. IGF-IR is also increased in GD-OFs compared with fibroblasts emanating from healthy orbital tissues (242–244). Monozygotic twin studies in patients with GD indicate that the skew toward IGF-IR+ lymphocytes cannot be attributed to heritable determinants but rather, appears to be acquired (245). PBMCs express and secrete several IGFBPs, which could enable their modulation of IGF-I actions (246, 247). This secretion is independent of the actions of GH or IGF-I. IGF-I release from these cells can be stimulated by GH (247). Taken together, these findings suggest that IGF-I can alter fundamental aspects of immune system function (217).
Evidence for cross-talk between GH-IGF and thyroid pathways
Complex interactions have been identified between the IGF-I and thyroid pathways (248). Intact function of both systems is required for normal body growth. Thyroid hormones act at multiple levels of the GH/IGF-I axis (248). For instance, T3 modulates pituitary secretion of GH and exerts influence on its peripheral actions (249–251). T3 acts through nuclear receptors to increase IGF-IR and IGF-I mRNA expression in rat pituitary cells (252, 253). IGF-I appears to inhibit T3-induced GH gene expression through a short, negative feedback loop (254). Circulating 3,5,3′,5′-tetraiodothyronine [thyroxine (T4)] levels are reduced following GH administration to GH-deficient adults. These individuals also exhibit blunted TSH response to TSH-releasing hormone. GH suppresses TSH through actions mediated by somatostatin (255–257) and appears to enhance T4 to T3 conversion, thereby promoting thyroid hormone actions at the target cell nucleus (257–259). Exogenous T4 stimulates IGF-I activity in the absence of GH in hypophysectomized or thyroidectomized animals (260). T3 induces cardiac IGF-I gene expression in hypophysectomized rats, whereas GH does not, suggesting that thyroid hormone plays a role in the autocrine/paracrine actions of IGF-I (261). Moreover, expression of IGF-I and IGF-IR is positively correlated with that of thyroid hormone receptor (TR)α1, TRα2, and TRβ in human heart atria, suggesting that levels of these genes may be functionally linked (262). On the other hand, TSH can stimulate human osteoblast-like cell proliferation and differentiation by upregulating IGFs and stimulatory IGFBPs, while downregulating inhibitory IGFBPs (263). Growth retardation in hypothyroid children appears to result from deficient production, secretion, and action of GH (264, 265). Both GH secretion and total serum IGF-I levels are decreased in hypothyroidism and are restored by thyroid hormone treatment (266). Furthermore, untreated hypothyroidism is associated with reduced systemic IGF-I bioactivity and decreased IGF-I actions on cartilage (264). GH replacement in hypopituitary animals or humans fails to normalize growth without normalization of the thyroidal state with T4 (266). Likewise, hyperthyroidism is also associated with decreased basal and stimulated GH release (267). Total (immunoreactive) IGF-I levels in hyperthyroidism have been reported as normal (251) or elevated (266, 268, 269); however, IGF-I bioactivity is markedly reduced in thyrotoxicosis when assessed by bioassays (189, 248, 266, 267, 270). This reduction might result from changes in IGFBP levels (248). Several mechanisms underlying this regulation by thyroid hormones have been proposed (248, 266, 271). T4 and/or T3 directly stimulate IGFBP expression in human hepatoma cells, potentially explaining elevated, circulating IGFBP-1 in thyrotoxicosis (266, 272–274). IGFBP-1 behaves as an inhibitor of IGF-I action in most bioassays. Elevated levels of this protein thus may be responsible for the markedly decreased IGF-I bioactivity observed in thyrotoxicosis (248, 266, 275, 276). After the euthyroid state is re-established, IGF-I bioactivity becomes normalized, as do several other abnormalities in the GH/IGF-I axis associated with thyrotoxicosis (248, 266, 277). IGF-I bioassays offer advantages over more commonly used, total IGF-I immunoassays, in that they selectively quantify active molecules (278). Disparate findings about the impact of thyrotoxicosis on IGF-I levels are likely to result from use of different types of assays in various studies (278–280). With regard to TAO, it remains likely that IGF-I bioassays reliably detect the activities of stimulating and inhibiting antibodies targeting IGF-IR.
Evidence for an interface between actions of IGF-I and TSH
Tramontano et al. (281) were among those to explore an intersection between the actions of IGF-I and TSH by studying their impact on the growth of Fischer rat thyroid cell line 5 cells. These cultures represent a nontransformed, established line of cloned rat thyroid follicular epithelial cells (282). Although IGF-I and TSH each enhances DNA synthesis and Fischer rat thyroid cell line 5 cell proliferation in an independent, dose-dependent manner, the combination of the two factors exhibits marked synergy (281). Remarkably, a similar synergy occurred when GD-IgG was added to IGF-I (281). As GD-IgG binds and activates TSHR, the authors concluded that thyroid epithelial cell growth regulation involved both IGF-IR and TSHR (281). The synergy existing between the actions of TSH and IGF-I was found to be mediated through 1,2-diacylglycerol (283). Considerably later, this same laboratory group demonstrated that bovine TSH and IGF-I synergistically increase HA accumulation in GD-OFs (284). Thus, the interplay between the pathways does not appear to be specific to thyroid tissue.
The functional implications surrounding interplay between TSHR and IGF-IR pathways were further explored in vivo when Clément et al. (285) determined that transgenic mice overexpressing both human IGF-I and IGF-IR selectively in the thyroid exhibited an increase in gland weight and enlarged follicular lumen area. Serum TSH levels in the transgenic animals were reduced, whereas T4 concentrations were found to be elevated, as was the uptake of iodine by the thyroid. These findings suggest that the coordinate overexpression of IGF-I and IGF-IR enhances the glandular sensitivity to the actions of TSH in vivo (285). In subsequent studies in vitro, Tsui et al. (286) reported that the rapid ERK phosphorylation resulting from treatment of primary human thyroid epithelial cells with rhTSH, IGF-I, or GD-IgG can be blocked by the monoclonal antibody IGF-IR inhibitor, 1H7 (Fig. 10). The authors therefore suggested that IGF-IR might mediate a component of signaling initiated through TSHR (286). A subsequent study revealed the impact of interrupting IGF-IR expression in vivo. Mice in which IGF-IR was conditionally knocked out in thyroid had substantially elevated serum TSH levels with decreased serum T4 and a reduction in monocarboxylate transporter 8 expression (287). Thyroid architecture was preserved, and gland size remained normal in these knockout animals compared with their controls (287). In another study, Müller et al. demonstrated that increased levels of TSH in conditionally knocked out IGF-IR−/− in the thyroid gland compensate for loss of the receptor protein in terms of thyroid hormone biogenesis while maintaining normal thyroid morphogenesis (288).
Figure 10.
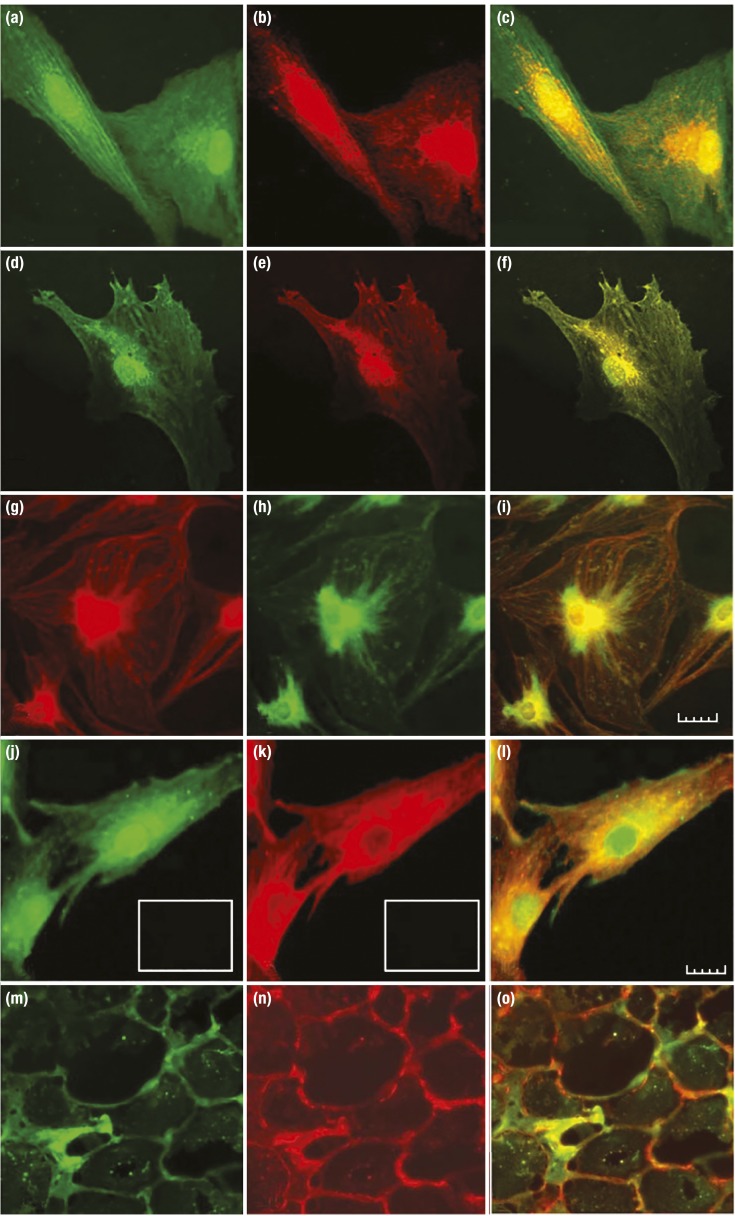
Evidence for the physical association of IGF-IR and TSHR in orbital tissues in situ, cultured OFs, and thyroid epithelial cells in vitro. (a–f) Immunofluorescence staining using an anti–IGF-IRβ antibody appearing as red and anti-TSHR antibody appearing as green by confocal microscopy. (a–c) OFs from a patient with TAO (GD-OFs). (d–f) Primary human thyrocytes. (g–i) Another set of antibodies is used where IGF-IRβ appears as green and TSHR as red. (j–l) TSHR is green, and IGF-IRα is red in GD-OFs. (m–o) TAO orbital tissue where TSHR is green, and IGF-IR is red. (c, f, i, l, and o) Merged images are captured. [Reproduced with permission from Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181(6):4397–4405. Copyright 2008 The American Association of Immunologists, Inc; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
TSH and IGF-I stimulate the nuclear accumulation of β-catenin in a Wnt-independent manner but act through distinct signaling mechanisms in cultured thyroid follicular cells (289). That study disclosed differential effects of the two molecules on cAMP/protein kinase A and PI3K activation. In aggregate, the results of these studies suggest strongly that the IGF-I pathway and IGF-IR, in particular, play important roles in the determination of the impact of TSH on the thyroid and in extrathyroidal tissues as well. They further divulged opportunities for indirect modification of both physiological and pathological signaling mediated through the TSH pathway.
Anti-IGF-IR antibodies and their potential role in TAO
Both naturally occurring antibodies directed against IGF-IR and those generated under experimental conditions are topics of substantial interest, both in defining the pathogenesis of disease and for developing treatment strategies. Circulating human antibodies typically have a half-life >1 week (290). Thus, stimulatory anti-IGF-IR antibodies may activate the receptor for a longer duration than that provoked by IGF-I, which exhibits half-life < 12 minutes (291). The number and affinity of ligand binding sites on IGF-IR possess an uncertain relationship with the magnitude and duration of antibody-mediated receptor activation (292). Most blocking antireceptor antibodies bind to the orthosteric site, attenuating further receptor activation. Allosteric antireceptor antibodies can also be generated. These do not associate with the ligand binding site but nonetheless, can activate signaling (290).
Much information about anti-IGF-IR antibodies and their potential relevance to TAO can be gleaned by inference from those immunoglobulins targeting IR in the context of diabetes mellitus. Orthosteric antibodies initiating IR signaling have been identified but are relatively uncommon (290, 293). In contrast, allosteric antibodies bind to a different site on the IR from the canonical insulin binding site (294). These allosteric antibodies exhibit distinct pharmacological profiles, ranging from partial agonists to antagonists (294). Allosteric antibodies activate IR by inducing intramolecular crosslinking of subunits rather than by interacting with specific epitopes (292). The combined actions of stimulatory allosteric antibodies and insulin result in additive effects (292). Furthermore, nonagonist allosteric anti-IR antibodies can increase the binding affinity of IR for insulin, up to 20-fold, by decreasing the dissociation rate and stabilizing the ligand-bound IR conformation (295). It remains uncertain whether IGF-IR subunit crosslinking occurs in an analogous manner.
Insinuation of anti-IGF-IR antibodies in the pathogenesis of TAO can be traced to the report of Weightman et al. (296), who demonstrated that GD-IgG could interact with IGF-I binding sites on GD-OFs, whereas immunoglobulins from healthy subjects did not. The authors found that GD-IgG displaced [125I] IGF-I from these cell-surface binding sites. Subsequently, Pritchard et al. (297) reported that GD-IgG could activate the FK506-binding protein 12–rapamycin-associated protein/mTOR/AKT/p70s6k pathway in GD-OFs. They also identified the cell-surface binding site for GD-IgG as IGF-IR (244) (Fig. 11). GD-OFs express higher IGF-IR levels than do those found in OFs from healthy individuals (244). Those studies also revealed that GD-IgG could induce IL-16, a CD4-specific chemoattractant, and RANTES, a C-C chemokine, in cultured GD-OFs (297) and primary human thyrocytes (298). Importantly, these actions of GD-IgG appeared to be mediated through pathways independent of TSHR and could be attenuated by dexamethasone and by rapamycin, a specific inhibitor of FK506-binding protein 12–rapamycin-associated protein/mTOR/p70s6k (298). Moreover, IH7 could also block the induction of cytokine expression and HA production by GD-IgG (120, 244). Dominant-negative IGF-IR transfected into the fibroblasts could also block the induction of these cytokines (244). Mice immunized with the TSHR A subunit generate detectable anti-IGF-IR antibodies (140, 141). It remains uncertain whether those antibodies found in TSHR-immunized mice or those generated in GD are active in initiating signaling through IGF-IR or whether TSIs fully account for the effects of GD-IgG on GD-OFs and thyroid epithelial cells reported (244, 297, 298). Varewijck et al. (189) reported that TAO serum could provoke IGF-IR tyrosine autophosphorylation using a well-validated and sensitive kinase IGF-IR activation assay. Levels of IGF-IR activation were lower than those found in the serum from healthy individuals. These findings are congruent with earlier studies (248, 266). Serum IGF-IR-stimulating activity declines with advancing age in healthy individuals. However, these authors unexpectedly found a positive correlation between IGF-IR activation and age in subjects with TAO (189, 280). Following immunoglobulin depletion, IGF-IR-stimulating activity was decreased in 50% of the samples (10/20) (189). The effect of immunoglobulin on IGF-IR activation was greatest in sera exhibiting high basal activity and decreased significantly following immunoglobulin depletion, suggesting that IGF-IR-stimulating immunoglobulins are present in a subset of patients with TAO. In aggregate, those findings support the concept that anti-IGF-IR antibodies are generated in GD. It is possible that IGF-IR activation varies among these antibodies. Stimulating, neutral, and blocking antibodies may compete for IGF-IR binding, thus modulating receptor activation. Should that prove to be the case, anti-IGF-IR antibody activity profiles might be analogous to those directed at TSHR.
Figure 11.
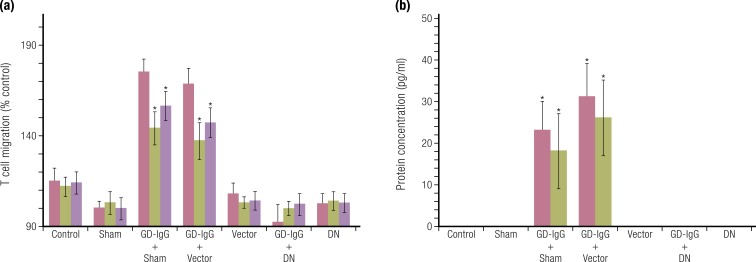
Evidence for specific activating antibodies recognizing IGF-IR in patients with GD. Expression of a dominant-negative (DN) mutant IGF-IR transfected into GD-OF blocks the induction by GD-IgG of (a) IL-16- and RANTES-dependent T cell chemoattraction and (b) protein expression. [Reproduced with permission from Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348–6354. Copyright 2003 The American Association of Immunologists, Inc.; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Results from other studies have not supported the concept of increased levels of stimulatory anti-IGF-IR antibodies in TAO. Minich et al. (299) investigated anti-IGF-IR antibodies in GD using another analytical method. They reported a similar prevalence of serum antibodies in individuals with TAO and in healthy controls (299). GD-IgG failed to stimulate IGF-IR autophosphorylation in Hep G2 cells but instead inhibited IGF-IR tyrosine phosphorylation. These GD-IgGs inhibited MCF7 breast cancer cell proliferation, leading the authors to conclude that the antibodies act as IGF-IR antagonists (299). In other studies, GD-IgG stimulated HA secretion in GD-OFs in the absence of detectable IGF-IR autophosphorylation (300). Two IGF-IR-blocking antibodies completely inhibited IGF-I-induced HA secretion in that study, leading the authors to conclude that IGF-IR is indirectly involved in the actions of GD-IgG. They found no evidence in patient-derived sera for IGF-IR-stimulating antibodies (300). Maximal induction of HA by GD-IgG in GD-OFs required metabolically active cells incubated in culture medium containing undetermined concentrations of IGF-I and IGF-II (284).
Factors underlying the divergent results obtained while examining GD-IgG actions mediated through IGF-IR remain uncertain. Use of multiple experimental conditions, nonstandardized study design, variable durations of treatment, different cellular targets and assays for detecting IGF-IR activation, and uncertain culture medium contents remain possible explanations. It is unlikely that any of the studies conducted in vitro are capable of fully recapitulating conditions existing in vivo, thus all of the studies reported, to date, are likely to have rendered nonphysiological results (1). Varewijck et al. (189) and Minich et al. (299) used receptor tyrosine phosphorylation as a surrogate of IGF-IR activation. In the former, an IGF-IR kinase IGF-IR activation reflected the integrated contributions of IGF-I, IGF-II, IGFBPs, and GD-IgG activities, whereas Minich et al. used purified GD-IgGs, excluding important serum factors that potentially influence cellular responses. Analogous to these issues, partial stimulatory effects were detected when IR activity was assessed following treatment with insulin and anti-IR monoclonal antibodies. Furthermore, IR stimulation was found to be restricted to a very narrow antibody concentration range, while antibody fragments were inactive (292). These issues may prove similar to those confronting the correct interpretation of findings regarding the activation by antibodies targeting IGF-IR in TAO. Relatively recent evidence that IGF-IR initiated signaling can involve events independent of the kinase domain. Thus, studies relying only on receptor tyrosine phosphorylation as readouts and excluding interactions with GPCRs could systematically fail to detect receptor involvement. In any event, the issue of stimulatory anti-IGF-IR antibodies as participating in the pathogenesis of TAO will require substantially more investigation before definitive conclusions can be drawn (301).
Interactions between IGF-IR and TSHR
A preliminary study demonstrated that anti-TSHR antibodies generated in GD could immunoprecipitate phosphorylated IGF-IRβ and IR, a finding suggesting that the three receptors might share common epitopes (302). These receptor proteins were subsequently shown to form physical and functional complexes in OFs and thyroid epithelial cells (286). Those later studies demonstrated further that TSHR signaling was dependent on the activity of IGF-IR. Treatment of primary human thyrocytes with rhTSH resulted in rapid ERK phosphorylation, which could be blocked by 1H7 (286) (Fig. 10). In pull-down studies involving fibroblasts, primary human thyrocytes, and human thyroid tissue, anti-IGF-IRβ and anti-TSHR antibodies brought both proteins out of solution (286). Moreover, IGF-IRβ and TSHR colocalized to the perinuclear and cytoplasmic compartments in cultured cells and in TAO orbital tissue in situ. In contrast, IGF-IRα exhibited a different pattern of subcellular localization (210, 286). Thus, when viewed in aggregate, these studies support the concept that IGF-IR functions as a critical molecular conduit for mediating components of TSHR downstream signaling (1). More recently, IGF-IR was found to form functional complexes with other GPCRs, suggesting that IGF-IR functions as a constituent of RTK/GPCR hybrids (196), integrating classical kinase signaling with canonical GPCR characteristics (196). These hybrids appear to broaden the array of downstream pathways relevant to IGF-IR signaling (196).
GPCRs and RTKs appear capable of reciprocal transactivation (303, 304). RTKs can engage GPCR and use their signaling molecules, including heterotrimeric G proteins and β-arrestins (303). This transactivation process, where GPCR signaling is downstream from that of RTK, can occur either following binding of a cognate GPCR ligand or may be ligand independent (303). Thus, RTKs can also act as “GPCR-like” receptors by virtue of “the company they keep” (196, 303). For example, IGF-IR can associate with Gai and Gbγ proteins, leading to activation of ERK and resulting in mitogenesis (305, 306). β-Arrestins, which serve as adaptors of GPCR endocytosis and transducers of GPCR signaling, can associate with IGF-IR in a ligand-dependent manner (307, 308). On the other hand, GPCR agonists can activate RTKs in the absence of activating ligands and, in part, by transactivation of GPCR (303, 304). Thus, a bidirectional crosscommunication between RTKs and GPCRs can occur (303, 304).
These RTK/GPCR interactions were confirmed recently regarding TSHR/IGF-IR when bidirectional receptor crosstalk was demonstrated in GD-OFs treated with TSH, IGF-I, and M22, a TSI (284). Those studies revealed that stimulation of HA could be partially inhibited by the small molecule IGF-IR inhibitor, linsitinib, and by the TSHR antagonist, C1 (284). The authors concluded that TSHR/IGF-IR interplay can occur, resulting in IGF-IR activation mediated through TSHR/IGF-IR crosstalk (284, 300). These studies have important limitations, as do virtually all reports concerning IGF-IR/TSHR interactions. They reveal an EC50 for IGF-I, ~300-fold below that of bTSH (IGF-I, 0.45 ± 0.15 nM vs TSH, 150 ± 21 nM) (284). IGF-I associates with human IGF-IR with a dissociation constant ~1 nM (188), whereas rhTSH binds TSHR with a dissociation constant ~ 0.3 nM (309, 310). Thus, bTSH concentrations used to stimulate HA accumulation were supraphysiological. bTSH actions were unaffected by cotreatment with IGF-I at physiological concentrations but were enhanced substantially (19-fold) when supraphysiologically IGF-I concentrations were used (284). The medium used in those studies contained 10% fetal bovine serum, in which nanomolar concentrations of IGF-I and IGF-II are present (311). Endogenous IGFs were likely contributing to HA production under control (untreated) culture conditions (284). It would appear that further studies, where experimental conditions are more precisely defined and standardized, will be necessary before the physiological relationships between IGF-IR and TSHR can be resolved and disparate findings, thus far reported, can be reconciled.
Current Therapeutic Environment for Active TAO
Absence of unambiguous effectiveness among therapies currently available to clinicians for active TAO has rendered care of many patients, especially those with the most severe disease, suboptimal. Moreover, no laboratory-based, objective biomarkers have been identified to aid in judging a clinical response. Scales for grading disease severity and activity are imperfect and the determination of whether TAO remains active or has transitioned to stable disease continues to challenge even the most skilled clinician.
Glucocorticoids are, by far, the most frequently used class of drugs in active TAO. They appear to benefit only ~50% of the patients to whom they are administered. Many reports have appeared over recent decades examining the use of glucocorticoids in active TAO and have attempted to identify the subsets of patients most likely to respond. Among these, the majority of studies have included insufficient numbers of patients to establish clearclinical benefit or to distinguish reliably the impact of the drug used from the natural course of the disease. A major barrier to implementing a definitive, adequately powered, properly controlled, and masked multicenter trial of steroids concerns the costs of such a study. That expense apparently has not been found to be justifiable by stakeholders given marketplace economics. Furthermore, many physicians remain reticent to allow their patients with severe disease to participate in placebo-controlled studies. Besides their uncertain benefit, glucocorticoids are associated with substantial, sometimes serious, side-effects (1). These include the worsening of glucose intolerance and diabetes mellitus, hypertension, osteoporosis, glaucoma, and psychiatric diseases (312). Several relatively recent reports have suggested that intravenous pulse therapy is superior to oral glucocorticoids in that it may be more effective and seems better tolerated with fewer side-effects. Among the most convincing and informative studies of glucocorticoids in TAO is that of Bartalena et al. (313). That study examined the effects on clinical activity scores (CAS) using three different cumulative dosages of intravenous methylprednisolone, administered as 12 weekly infusions (2.25, 4.98, and 7.47 g in total). It demonstrated transient benefit, the greatest in the group receiving the highest dosage after a 12-week treatment period. There was a clinically inconsequential reduction of 0.6 mm proptosis in that treatment group compared with baseline. But, high-dosage intravenous steroids can result in severe liver dysfunction (314). Clearly, patients with pre-existing liver abnormalities, such as viral hepatitis and alcoholic liver disease, are at substantially higher risk for liver failure following high-dose steroids. A careful inventory of those antecedent factors associated with liver toxicity should be carefully undertaken before the use of pulse IV glucocorticoids in TAO.
Some experts continue to advocate the use of external beam radiotherapy, alone or in combination with steroids (315). Among those to consider the combination of orbital radiotherapy and systemic glucocorticoids were Marcocci et al. (316) and Pinchera et al. (317). This has been examined in several studies (318). The issue of radiotherapy in benign diseases, such as TAO, continues to generate substantial debate. Over four decades of experience with external beam radiotherapy in TAO has yielded mixed views about its rightful place in routine disease management (319–323). In addition to serious doubts about efficacy (324), side-effects, such as radiation-induced retinopathy, were recognized early and remain concerning (325, 326). The induction of tumors represents a theoretical risk (327). Long-term follow-up has not disclosed increased cancer incidence, but the cohort of patients subjected to decades of surveillance, thus far, is probably inadequate to come to definitive conclusions (328, 329). Special concern for its use in patients with diabetes has been articulated (330). These relate to the potential negative impact on retinal vascular disease. When compared with systemic glucocorticoids, the responses to radiotherapy are often found to be equivalent (331, 332). The combination of the two modalities may be more effective than either as a single therapy (333). Radiotherapy may improve surgical decompression outcomes related to extraocular muscle volumes and diplopia (315).
In addition to glucocorticoids, a limited array of biologicals has been examined, largely in small, single institution pilot studies. Some of these agents have shown clinical benefit in other autoimmune diseases and are now under consideration for repurposing to TAO. Among the most widely studied is the CD20-targeting monoclonal antibody, rituximab (334). This drug was originally developed for the treatment of B cell lymphoma but has been found to be effective in several autoimmune diseases, such as multiple sclerosis, RA, systemic lupus erythematosus, and neuromyelitis optica spectrum disorder (335). B Cell depletion in GD was initially considered several years ago. Early studies examined responses in terms of improved hyperthyroidism and TAO (336–338). Two recent, contemporaneously reported clinical trials in patients with TAO came to very different conclusions. Both were conducted in single institutions, were prospective, and double masked. Both included a 24-week treatment period and lacked lengthy follow-up periods. One, comparing rituximab to methylprednisolone, found greater improvement in the CAS in those patients receiving rituximab (339). The other, a placebo-controlled study, found no difference in disease activity following treatment with the active drug (340). Neither study revealed meaningful improvement in proptosis in any treatment group.
Anticytokine drugs, including those agents directed at TNF-α and IL-6, have not yet undergone evaluation in human subjects with TAO, or the studies have been extremely limited (341). The rationale for examining these agents derives from the putative involvement of their respected pathways in the disease (72, 342). Results from these small studies are inadequate to judge whether the general strategy of targeting cytokines will yield effective therapy. Furthermore, the road to therapy for TAO with cytokine-targeting molecules might be complicated, as is suggested by the emergence of thyroid autoimmunity following administration of anti-TNF-α antibodies for RA (343).
A very recent study of 164 patients with active, moderate-to-severe TAO failed to demonstrate convincingly the additional benefit of adding mecophenolate to treatment with methylprednisolone compared with the steroid alone (344).
“Teprotumumab was highly effective in reducing proptosis, CAS, QOL, and diplopia in active, moderate to severe disease compared to placebo.”
In sum, currently available therapies for active TAO are viewed widely as inadequate and have proven to be ineffective in many patients, especially those with particularly severe disease. Moreover, most of the commonly used modalities, such as systemic glucocorticoids, external beam radiation, and the combination of the two, are fraught with potentially serious side-effects that limit their dosage and duration of treatment. Other, more recently introduced approaches, such as B cell depletion and anticytokine agents, have yet to be proven safe and effective, despite their more targeted nature. None has thus far been shown to alter meaningfully the natural course of TAO or to lessen the need for surgical remediation. Thus the need for improved options for treating the disease persists.
Provenance for IGF-IR as a Therapeutic Target for Human Disease
IGF-IR as a therapeutic target in cancer
The widespread involvement of the IGF-I pathway in the regulation of multiple physiological functions renders its components worthy of consideration as potential therapeutic targets for human disease. Whereas the earliest clinical translation of emerging insight into the nature of this pathway concerned treatment of growth retardation, other disease states soon became the focus of investigation. Relatively soon after its identification and molecular cloning, IGF-IR was proposed as a potentially important therapeutic target in cancer (345). Extensive mechanistic links between cancer and the IGF-I pathway have been identified. These emanated from early observations concerning the increased incidence of tumors in states of GH and IGF-I excess, such as acromegaly (346, 347). IGF-IR appears to be required for malignant transformation in some cell types (348). Several drug developmental programs were initiated independently at multiple pharmaceutical companies aimed at the generation of IGF-IR inhibitors. These efforts culminated in molecules that underwent testing for their efficacy in many types of cancer in several clinical trials. Among these molecules were several IGF-IR-blocking antibodies (349). Some of these molecules were thought to act through the IGF-I binding site and induce internalization/degradation of the receptor, a mechanism common to most candidates (350). Subsequently, it was found that antibody-provoked IGF-IR internalization can result in activation of IGF-IR/β-arrestin-1-mediated ERK signaling (197). Thus, the actions of these blocking antibodies can be mediated through at least two distinct mechanisms; while blocking the receptor, they can also function as IGF-IR/β-arrestin-1-biased agonists (197). These findings suggest that naturally occurring IGF-IR-blocking antibodies could also stimulate post-IGF-IR signaling and therefore, could participate in the development of diseases, such as TAO.
Rationale for therapeutically targeting IGF-IR in TAO
As reviewed earlier, a number of intersecting aspects of IGF-IR with the pathogenesis of TAO suggested that the interruption of this pathway might alter the clinical behavior of the disease. GD-IgGs can bind and activate IGF-IR signaling (120, 189, 244, 286, 296). IGF-IR levels are elevated in GD-OFs and T cells from these patients, whereas the abundance of IGF-IR-displaying B cells is increased in the disease (242–244, 286). Furthermore, IGF-IR serves as a downstream target for activities initiated through TSHR (286). IGF-I levels are elevated in both orbital fat and muscle in TAO (351, 352), potentially resulting from local IGF-I production by GD-OF (353). Thus, it appeared likely that the IGF-I pathway plays an important role in the pathogenesis of TAO, both through intrinsic properties, resulting in autoantibody-activated signaling, and through autocrine/paracrine mechanisms. These findings prompted the organization of clinical studies to determine whether IGF-IR could be successfully targeted as therapy.
Phase II therapeutic trial of teprotumumab in TAO
A recently completed clinical trial examining the potential clinical benefit and safety of the IGF-IR-inhibiting monoclonal antibody, teprotumumab, has revealed unprecedented effectiveness in active, moderate-to-severe TAO (354) (Fig. 12). The study was organized by and participated in by T.J.S. and was predicated entirely on in vitro studies examining the interactions among GD-IgGs and GD-OFs and fibrocytes (120, 244, 286, 297, 355) and evidence that TSHR and IGF-IR form a physical and functional signaling complex (286). Teprotumumab is a fully human monoclonal antibody of the IgG1 class that acts by binding to the cysteine-rich region of the ligand-binding pocket in the extracellular domain of IGF-IR. This binding results in the loss of the receptor/antibody complex from the cell surface and its entrance into intracellular degradation pathways. Importantly, teprotumumab does not exhibit any avidity toward IR. Enrollment in the double-masked, placebo-controlled, randomized trial commenced on 2 July 2013 and was completed 23 September 2015 at 15 performance sites in North America and Europe. A standardized protocol was adhered to throughout the study. Patients were assigned randomly to either a group receiving placebo or to the treatment arm receiving active drug at a ratio of 1:1. Inclusion criteria included development of TAO within 9 months of enrollment in patients between 18 and 75 years of age. Their disease must have been judged as active, manifesting a CAS greater than or equal to four using a seven-point scale, where CAS greater than or equal to three indicates active disease in the study (more severely affected) eye. Study participants could not have undergone either specific medical (such as B cell depletion) or surgical treatment in the past for their TAO. Individuals having received high-dose glucocorticoids previously were excluded, unless the total dosage was 1 g or less and a 3-month washout period had elapsed.
Figure 12.
Screening, randomization, response, and follow-up of patients’ participation in clinical trial RV001. (a) Patients meeting inclusion criteria entered the trial-screening process. At baseline, patients were randomized to receive active drug or placebo for the 24-week intervention phase. This was followed by a 1-year observation. (b) An analysis to first response. (c) The time course of patients meeting response criteria. (d) Responses are graded at week 24, where a high response indicates ≥3 mm proptosis and clinical activity score (CAS) reduced greater than or equal to three points on a seven-point scale. [Reproduced with permission from Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. Copyright Massachusetts Medical Society; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Study outcomes included the primary efficacy aggregate endpoint of both improved CAS greater than or equal to two points and a reduction in proptosis of ≥2 mm, both in the study eye, in the absence of a similar magnitude of worsening in the fellow (less severely affected) eye. Secondary efficacy endpoints measured as continuous independent variables over time included improvement in CAS greater than or equal to two points and reduction in proptosis ≥2 mm from baseline. QOL was assessed using a validated survey instrument [Graves Ophthalmopathy (GO)--QOL] with subscales for visual function and appearance, and subjective diplopia was also quantified. A total of 112 patients underwent screening, of which 88 then were randomized to one of the treatment groups: 45 receiving placebo and 42 who were administered active drug (Fig. 12a).
Effectiveness
Figure 12b demonstrates analysis to first response, and Fig. 12c shows the time course of patients who responded to teprotumumab compared with those in the placebo group. Highly significant differences between groups could be detected by week 6 of the treatment period (P < 0.001). When the primary response was graded at week 24, the dramatic differences continued. A high response (CAS improvement greater than or equal to three points and proptosis reduction ≥3 mm) was observed in a subset of patients receiving active drug vs placebo (P < 0.001; Fig. 12d). Secondary efficacy end points, including changes in proptosis and in CAS from baseline demonstrated the significant differences between treatment groups at 6 weeks (P < 0.001), and these were maintained or increased at every assessment thereafter (all P < 0.001) through week 24 (Fig. 13b and 13c). Other secondary endpoints, including GO-QOL visual functioning subscale and subjective diplopia, showed significant improvements in the patients receiving teprotumumab compared with placebo (Fig. 13d–13f).
Figure 13.
Secondary efficacy end points in clinical trial RV001. (a) The time course of changes in proptosis from baseline. (b) The time course of change from baseline in CAS. (c) Post hoc analysis of the fraction of patients with CAS of zero or one at the time point indicated along the abscissa. (c) Change in visual functioning subscale of the QOL scale (GO-QOL). (d) GO-QOL visual-functioning subscale. (e) Change in appearance subscale of GO-QOL. (f) Responses in terms of diplopia. [Reproduced with permission from Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. Copyright Massachusetts Medical Society; © 2019 Illustration Presentation ENDOCRINE SOCIETY].
Safety profile of teprotumumab in TAO
The safety profile of teprotumumab in the trial was encouraging and was considerably less concerning than that found in earlier clinical studies of teprotumumab in cancer patients (356). In the TAO trial, six subjects in each group discontinued treatment; five of those receiving teprotumumab experienced an adverse event. The only adverse event that could be attributed unambiguously to the drug was worsening hyperglycemia, mainly in a few patients with pre-existing diabetes. This was easily managed with increases in diabetes medication. In each case, these medications could be reduced to levels used before study participation after the therapy phase was completed. Other side-effects seen in the earlier cancer studies were not detected in the TAO study, such as thrombocytopenia, fatigue, and anemia.
Trial summary
In this initial clinical trial examining IGF-IR inhibition in TAO, teprotumumab was highly effective in reducing proptosis, CAS, QOL, and diplopia in active, moderate-to-severe disease compared with placebo. The effects on proptosis were unprecedented for any medical treatment and appear to be equivalent to the best outcomes reported following surgical remediation. Responses to teprotumumab were rapid, and differences in the primary outcome between treatment groups were highly substantial within 6 weeks of the initial infusion. Durability of the therapeutic signal is currently being monitored in a follow-up phase. Because no orbital imaging was performed before and following the therapy, it is uncertain whether the reduction in proptosis was a consequence of volume reduction of fat, extraocular muscles, or both. The US Food and Drug Administration has designated teprotumumab as “breakthrough” therapy for moderate-to-severe, active TAO, based on the results of the initial trial.
The trial had several limitations. Despite attempts to stratify for smoking status, an imbalance with regard to this variable existed between the treatment groups. As stated above, the study did not include pre- and post-treatment orbital imaging, and thus, it remains uncertain whether the treatment affected the muscle, connective tissue, or both tissue compartments. The trial was limited to early (<9 month duration), active disease. Thus, it remains uncertain whether teprotumumab might also be effective in more established disease, perhaps in individuals who have already entered the stable phase.
Current phase III trial of teprotumumab
A confirmatory phase III trial has been organized and is currently enrolling at several performance sites throughout North America and Europe (target 76 patients). The study, like its predecessor, is double masked, multicenter, randomized (1:1 ratio), and stratified by tobacco use to either teprotumumab or placebo arm. As was the case with the first trial, patients must be within 9 months of the initial presentation of their symptoms and signs of moderate-to-severe TAO. They must exhibit disease activity (at least four points on a zero to seven point scale, where a score of greater than or equal to three points indicates active TAO) at the time of study entry. The treatment phase is 24 weeks in duration, during which eight infusions are administered at 3-week intervals. The primary response outcome is effect of teprotumumab vs placebo on the proptosis responder rate (≥2 mm) in the study eye at 24 weeks. Secondary outcomes include the following (1): the aggregate of greater than or equal to two-point reduction in a seven-point CAS scale and ≥2 mm proptosis from baseline in the study eye at week 24 (2); percentage of patients with CAS of zero or one in the study eye at week 24 (3); mean change from baseline of proptosis in the study eye at week 24 (4); mean change from baseline in GO-QOL at week 24. At week 24, the end of the treatment period, those patients not responding (i.e., those with <2 mm of proptosis reduction from baseline) will be eligible to enter an open-label study in which all nonresponders will be offered teprotumumab. Following the treatment phase, study patients will enter a 48-week follow-up observation period.
Potential use of teprotumumab in stable and mild TAO
The recently concluded study and the current trial were both designed to examine the effectiveness and safety of IGF-IR inhibition in active TAO. Neither could address the issue of whether this therapeutic strategy might also be effective in stable disease. Follow-up studies will be necessary to evaluate whether teprotumumab is effective and safe in patients with inactive disease. Given the unanticipated and dramatic reversal of proptosis found in the recently concluded study (354), it is possible that teprotumumab might benefit patients with tissue changes characteristic of stable disease.
Potential targeting IGF-IR in other autoimmune diseases
The impact of the IGF-I/IGF-IR/IGFBP pathway on the regulation of host immunity, tissue remodeling, and inflammation suggests its potential participation in autoimmune diseases besides TAO. Given the consequences of IGF-IR interruption on the thyroid response to TSH (287), it is possible that teprotumumab or a similarly acting drug might therapeutically block the actions of TSIs and therefore, reduce glandular overactivity in GD. It should be noted that both studies, thus far implemented for TAO, have excluded hyperthyroid patients, and thus, this issue remains unexplored.
Critical to exploring the potential for therapeutically targeting IGF-IR in other diseases will be the identification of those pathogenic mechanisms shared by multiple forms of autoimmunity that involve this pathway. A disease likely to have a similar set of therapeutic responses to teprotumumab or other inhibitors of IGF-IR is RA. Pritchard et al. (357) found equivalent responses of synovial fibroblasts from patients with that disease to IGF-I, sera, and IgGs from these same individuals. These receptor agonists induced the expression of IL-16 and RANTES and provoked the generation of chemoattractant signals in RA synovial fibroblasts. More recent studies suggest that TNF-α can induce IGF-I and IGFBP3 expression in synovial fibroblasts and that intrasynovial macrophages from patients with RA generate IGFBP3 (358). Small molecule tyrosine kinase inhibitors have been shown to inhibit osteoclastogenesis, a component of RA (359) and the development of synovitis (358). Thus, several processes involved in the development of RA involve the IGF-I pathway, identifying a plausible extension of the strategies used in teprotumumab-treated TAO. Studies are currently underway in the T.J.S. laboratory examining the cell populations being targeted by teprotumumab, including those derived from within the orbit and those functioning systemically.
Conclusions
The pathogenesis of TAO, like most autoimmune diseases, remains shrouded in uncertainty. Underpinning the disease is the loss of immune tolerance to the central antigen, TSHR. The continued reliance on immunosuppressive drugs, even those with relative specificity, can be associated with substantial adverse events reflecting crippled host defense, such as infections and opportunistic cancers. Involvement of IGF-IR as an autoantigen may represent a primary aspect of disease development or could result secondarily, such as would be the case following epitope spreading. Recognition of the intimate relationship between TSHR and IGF-IR has allowed the emergence of a therapeutic approach that is potentially paradigm shifting. The apparent effectiveness of teprotumumab and its promising side-effect profile makes IGF-IR inhibition an exciting, early step on the road to treatments that can offer patients improved QOL in relative safety. The apparent involvement of the IGF-I pathway in TAO may merely represent an example of a more-generalizable relationship with human autoimmune diseases. The recognition of the broader role of IGF-IR in regulating both normal and pathological immune responses may offer important opportunities for therapeutic intervention in several allied diseases that have proven particularly difficult to treat (360). Thus, the targeting of IGF-IR by teprotumumab or other IGF-IR inhibiting agents, including monoclonal antibodies and small molecules, may have therapeutic applicability in other autoimmune diseases. Further studies will be required to clarify unambiguously whether IGF-IR-targeted therapy is effective and carries an acceptable profile of adverse events in these diseases.
Acknowledgments
The authors are grateful to Ms. Linda Polonsky for her outstanding suggestions as we wrote this manuscript and to Jeffrey M. Korff for superb editorial suggestions. The expert editorial support provided by Ms. Darla Kroft is gratefully acknowledged.
Financial Support: This work was supported, in part, by US National Institutes of Health Grants EY008976, EY11708, DK063121, and 5UMIA110557; a Core Center for Research Grant EY007003 from the National Eye Institute; an unrestricted grant from the Research to Prevent Blindness; and the Bell Charitable Family Foundation.
Disclosure Summary: T.J.S. has been issued patents covering his inventions concerning the use of IGF-IR inhibitors as therapy in Graves disease. These patents are held by UCLA School of Medicine and Los Angeles Biomedical Research Institute. J.A.M.J.L.J. has nothing to disclose.
Glossary
Abbreviations
- AIRE
autoimmune regulator protein
- bTSH
bovine thyrotropin
- CAS
clinical activity score
- CD40L
CD40 ligand
- GD
Graves disease
- GO
Graves ophthalmopathy
- GPCR
G-protein-coupled receptor
- HA
hyaluronan
- IGF-I/IIR
insulin-like growth factor I/II receptor
- IGFBP
insulin-like growth factor-binding protein
- IR
insulin receptor
- IRS
insulin receptor substrate
- MHC-II
major histocompatibility complex class II
- OF
orbital fibroblast
- p70S6k
phosphoprotein 70 ribosomal protein S6 kinase
- PBMC
peripheral blood mononuclear cell
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PI3K
PPARγ, proliferator-activated receptor γ
- QOL
quality of life
- RA
rheumatoid arthritis
- RAI
radioactive iodine
- RANTES
regulated on activation, normal T expressed and secreted
- rh
recombinant human
- RTK
receptor tyrosine kinase
- T3
3,5,3′-triiodothyronine
- T4
3,5,3′,5′-tetraiodothyronine (thyroxine)
- TAO
thyroid-associated ophthalmopathy
- Th
T helper
- TR
thyroid hormone receptor
- TSHR
thyrotropin receptor
- TSI
thyroid-stimulating immunoglobulin
References
- 1. Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552–1565. [DOI] [PubMed] [Google Scholar]
- 2. Nordyke RA, Gilbert FI Jr, Harada AS. Graves’ disease. Influence of age on clinical findings. Arch Intern Med. 1988;148(3):626–631. [DOI] [PubMed] [Google Scholar]
- 3. Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM; European Group on Graves’ Orbitopathy (EUGOGO) . The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan W, Wong GW, Fan DS, Cheng AC, Lam DS, Ng JS. Ophthalmopathy in childhood Graves’ disease. Br J Ophthalmol. 2002;86(7):740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Putta-Manohar S, Perros P. Epidemiology of Graves’ orbitopathy. Pediatr Endocrinol Rev. 2010;7(Suppl 2):182–185. [PubMed] [Google Scholar]
- 6. Perros P, Žarković M, Azzolini C, Ayvaz G, Baldeschi L, Bartalena L, Boschi A, Bournaud C, Brix TH, Covelli D, Ćirić S, Daumerie C, Eckstein A, Fichter N, Führer D, Hegedüs L, Kahaly GJ, Konuk O, Lareida J, Lazarus J, Leo M, Mathiopoulou L, Menconi F, Morris D, Okosieme O, Orgiazzi J, Pitz S, Salvi M, Vardanian-Vartin C, Wiersinga W, Bernard M, Clarke L, Currò N, Dayan C, Dickinson J, Knežević M, Lane C, Marcocci C, Marinò M, Möller L, Nardi M, Neoh C, Pearce S, von Arx G, Törüner FB. PREGO (presentation of Graves’ orbitopathy) study: changes in referral patterns to European Group On Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br J Ophthalmol. 2015;99(11):1531–1535. [DOI] [PubMed] [Google Scholar]
- 7. Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. The incidence of Graves’ ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120(4):511–517. [DOI] [PubMed] [Google Scholar]
- 8. Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Kendall-Taylor P, Perros P, Neoh C, Dickinson AJ, Lazarus JH, Lane CM, Heufelder AE, Kahaly GJ, Pitz S, Orgiazzi J, Hullo A, Pinchera A, Marcocci C, Sartini MS, Rocchi R, Nardi M, Krassas GE, Halkias A. Multi-center study on the characteristics and treatment strategies of patients with Graves’ orbitopathy: the first European Group on Graves’ Orbitopathy experience. Eur J Endocrinol. 2003;148(5):491–495. [DOI] [PubMed] [Google Scholar]
- 9. Smith TJ. Pathogenesis of Graves’ orbitopathy: a 2010 update. J Endocrinol Invest. 2010;33(6):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNab AA. The 2017 Doyne Lecture: the orbit as a window to systemic disease. Eye (Lond). 2018;32(2):248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3-4):177–194. [PubMed] [Google Scholar]
- 12. Zouvelou V, Potagas C, Karandreas N, Rentzos M, Papadopoulou M, Zis VP, Vassilopoulos D. Concurrent presentation of ocular myasthenia and euthyroid Graves ophthalmopathy: a diagnostic challenge. J Clin Neurosci. 2008;15(6):719–720. [DOI] [PubMed] [Google Scholar]
- 13. Novaes P, Diniz Grisolia AB, Smith TJ. Update on thyroid-associated ophthalmopathy with a special emphasis on the ocular surface. Clin Diabetes Endocrinol. 2016;2(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol. 2000;84(6):600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soares-Welch CV, Fatourechi V, Bartley GB, Beatty CW, Gorman CA, Bahn RS, Bergstralh EJ, Schleck CD, Garrity JA. Optic neuropathy of Graves disease: results of transantral orbital decompression and long-term follow-up in 215 patients. Am J Ophthalmol. 2003;136(3):433–441. [DOI] [PubMed] [Google Scholar]
- 17. Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond). 2007;21(9):1135–1145. [DOI] [PubMed] [Google Scholar]
- 18. Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79(2):542–546. [DOI] [PubMed] [Google Scholar]
- 19. Stan MN, Durski JM, Brito JP, Bhagra S, Thapa P, Bahn RS. Cohort study on radioactive iodine-induced hypothyroidism: implications for Graves’ ophthalmopathy and optimal timing for thyroid hormone assessment. Thyroid. 2013;23(5):620–625. [DOI] [PubMed] [Google Scholar]
- 20. Fernando R, Placzek E, Reese EA, Placzek AT, Schwartz S, Trierweiler A, Niziol LM, Raychaudhuri N, Atkins S, Scanlan TS, Smith TJ. Elevated serum tetrac in Graves disease: potential pathogenic role in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2017;102(3):776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hegedüs L, Bonnema SJ, Smith TJ, Brix TH. Treating the thyroid in the presence of Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):313–324. [DOI] [PubMed] [Google Scholar]
- 22. Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf). 2008;69(6):943–950. [DOI] [PubMed] [Google Scholar]
- 23. Träisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, Hallengren B, Hedner P, Lantz M, Nyström E, Ponjavic V, Taube A, Törring O, Wallin G, Asman P, Lundell G; Thyroid Study Group of TT 96 . Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94(10):3700–3707. [DOI] [PubMed] [Google Scholar]
- 24. Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989;321(20):1349–1352. [DOI] [PubMed] [Google Scholar]
- 25. Witte J, Goretzki PE, Dotzenrath C, Simon D, Felis P, Neubauer M, Röher HD. Surgery for Graves’ disease: total versus subtotal thyroidectomy-results of a prospective randomized trial. World J Surg. 2000;24(11):1303–1311. [DOI] [PubMed] [Google Scholar]
- 26. Järhult J, Rudberg C, Larsson E, Selvander H, Sjövall K, Winsa B, Rastad J, Karlsson FA, Group TEOS; TEO Study Group . Graves’ disease with moderate-severe endocrine ophthalmopathy-long term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid. 2005;15(10):1157–1164. [DOI] [PubMed] [Google Scholar]
- 27. Simmonds MJ. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol. 2013;9(5):277–287. [DOI] [PubMed] [Google Scholar]
- 28. Brix TH, Kyvik KO, Christensen K, Hegedüs L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86(2):930–934. [DOI] [PubMed] [Google Scholar]
- 29. Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85(2):536–539. [DOI] [PubMed] [Google Scholar]
- 30. Jin Y, Teng W, Ben S, Xiong X, Zhang J, Xu S, Shugart YY, Jin L, Chen J, Huang W. Genome-wide scan of Graves’ disease: evidence for linkage on chromosome 5q31 in Chinese Han pedigrees. J Clin Endocrinol Metab. 2003;88(4):1798–1803. [DOI] [PubMed] [Google Scholar]
- 31. Brown RS, Lombardi A, Hasham A, Greenberg DA, Gordon J, Concepcion E, Hammerstad SS, Lotay V, Zhang W, Tomer Y. Genetic analysis in young-age-of-onset Graves’ disease reveals new susceptibility loci. J Clin Endocrinol Metab. 2014;99(7):E1387–E1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D, Chen J, Zhang H, Zhang F, Yang L, Mou Y. Role of different CD40 polymorphisms in Graves’ disease and Hashimoto’s thyroiditis. Immunol Invest. 2017;46(6):544–551. [DOI] [PubMed] [Google Scholar]
- 33. Briones-Urbina R, Bear JC, Farid NR. Association of hypergammaglobulinemia G with HLA-DR3 in Graves’ disease. Tissue Antigens. 1982;19(1):20–24. [DOI] [PubMed] [Google Scholar]
- 34. Xiong H, Wu M, Yi H, Wang X, Wang Q, Nadirshina S, Zhou X, Liu X. Genetic associations of the thyroid stimulating hormone receptor gene with Graves diseases and Graves ophthalmopathy: a meta-analysis. Sci Rep. 2016;6:30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lombardi A, Menconi F, Greenberg D, Concepcion E, Leo M, Rocchi R, Marinó M, Keddache M, Tomer Y. Dissecting the genetic susceptibility to Graves’ disease in a cohort of patients of Italian origin. Front Endocrinol (Lausanne). 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pujol-Borrell R, Giménez-Barcons M, Marín-Sánchez A, Colobran R. Genetics of Graves’ disease: special focus on the role of TSHR gene. Horm Metab Res. 2015;47(10):753–766. [DOI] [PubMed] [Google Scholar]
- 37. Stefan M, Wei C, Lombardi A, Li CW, Concepcion ES, Inabnet WB III, Owen R, Zhang W, Tomer Y. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc Natl Acad Sci USA. 2014;111(34):12562–12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomer Y, Hasham A, Davies TF, Stefan M, Concepcion E, Keddache M, Greenberg DA. Fine mapping of loci linked to autoimmune thyroid disease identifies novel susceptibility genes. J Clin Endocrinol Metab. 2013;98(1):E144–E152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Südhof TC. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell. 2017;171(4):745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Rønningen KS, Guja C, Ionescu-Tîrgovişte C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. [DOI] [PubMed] [Google Scholar]
- 41. Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J Clin Endocrinol Metab. 1995;80(1):41–45. [DOI] [PubMed] [Google Scholar]
- 42. Heward JM, Brand OJ, Barrett JC, Carr-Smith JD, Franklyn JA, Gough SC. Association of PTPN22 haplotypes with Graves’ disease. J Clin Endocrinol Metab. 2007;92(2):685–690. [DOI] [PubMed] [Google Scholar]
- 43. Kurylowicz A, Kula D, Ploski R, Skorka A, Jurecka-Lubieniecka B, Zebracka J, Steinhof-Radwanska K, Hasse-Lazar K, Hiromatsu Y, Jarzab B, Bednarczuk T. Association of CD40 gene polymorphism (C-1T) with susceptibility and phenotype of Graves’ disease. Thyroid. 2005;15(10):1119–1124. [DOI] [PubMed] [Google Scholar]
- 44. Lin JD, Yang SF, Wang YH, Fang WF, Lin YC, Lin YF, Tang KT, Wu MY, Cheng CW. Analysis of associations of human BAFF gene polymorphisms with autoimmune thyroid diseases. PLoS One. 2016;11(5):e0154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin JD, Yang SF, Wang YH, Fang WF, Lin YC, Liou BC, Lin YF, Tang KT, Cheng CW. Associations of melatonin receptor gene polymorphisms with Graves’ disease. PLoS One. 2017;12(9):e0185529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong KH, Rong SS, Chong KK, Young AL, Pang CP, Chen LJ. Genetic associations of interleukin-related genes with Graves’ ophthalmopathy: a systematic review and meta-analysis. Sci Rep. 2015;5(1):16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9(1):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coppedè F. Epigenetics and autoimmune thyroid diseases. Front Endocrinol (Lausanne). 2017;8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Binder G, Mavridou K, Wollmann HA, Eggermann T, Ranke MB. Screening for insulin-like growth factor-I receptor mutations in patients with Silver-Russell syndrome. J Pediatr Endocrinol Metab. 2002;15(8):1167–1171. [DOI] [PubMed] [Google Scholar]
- 50. Bonafè M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88(7):3299–3304. [DOI] [PubMed] [Google Scholar]
- 51. San Millán JL, Cortón M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J Clin Endocrinol Metab. 2004;89(6):2640–2646. [DOI] [PubMed] [Google Scholar]
- 52. Garcia J, Ahmadi A, Wonnacott A, Sutcliffe W, Nagga K, Soderkvist P, Marcusson J. Association of insulin-like growth factor-1 receptor polymorphism in dementia. Dement Geriatr Cogn Disord. 2006;22(5-6):439–444. [DOI] [PubMed] [Google Scholar]
- 53. Chen C, Freeman R, Voigt LF, Fitzpatrick A, Plymate SR, Weiss NS. Prostate cancer risk in relation to selected genetic polymorphisms in insulin-like growth factor-I, insulin-like growth factor binding protein-3, and insulin-like growth factor-I receptor. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2461–2466. [DOI] [PubMed] [Google Scholar]
- 54. Stanilova SA, Ivanova MG, Karakolev IA, Stoilov RM, Rashkov RK, Manolova IM. Association of +3179G/A insulin-like growth factor-1 receptor polymorphism and insulin-like growth factor-1 serum level with systemic lupus erythematosus. Lupus. 2013;22(13):1388–1393. [DOI] [PubMed] [Google Scholar]
- 55. Weiss M, Ingbar SH, Winblad S, Kasper DL. Demonstration of a saturable binding site for thyrotropin in Yersinia enterocolitica. Science. 1983;219(4590):1331–1333. [DOI] [PubMed] [Google Scholar]
- 56. Heyma P, Harrison LC, Robins-Browne R. Thyrotrophin (TSH) binding sites on Yersinia enterocolitica recognized by immunoglobulins from humans with Graves’ disease. Clin Exp Immunol. 1986;64(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- 57. Rajendram R, Bunce C, Adams GG, Dayan CM, Rose GE. Smoking and strabismus surgery in patients with thyroid eye disease. Ophthalmology. 2011;118(12):2493–2497. [DOI] [PubMed] [Google Scholar]
- 58. Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W; European Group on Graves’ Orbitopathy . Selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364(20):1920–1931. [DOI] [PubMed] [Google Scholar]
- 59. Dehina N, Hofmann PJ, Behrends T, Eckstein A, Schomburg L. Lack of association between selenium status and disease severity and activity in patients with Graves’ ophthalmopathy. Eur Thyroid J. 2016;5(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laurberg P, Jørgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carlé A, Vejbjerg P. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006;155(2):219–228. [DOI] [PubMed] [Google Scholar]
- 61. Yamashita H, Noguchi S, Takatsu K, Koike E, Murakami T, Watanabe S, Uchino S, Yamashita H, Kawamoto H. High prevalence of vitamin D deficiency in Japanese female patients with Graves’ disease. Endocr J. 2001;48(1):63–69. [DOI] [PubMed] [Google Scholar]
- 62. Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphism is associated with Graves’ disease in the Japanese population. J Clin Endocrinol Metab. 2000;85(12):4639–4643. [DOI] [PubMed] [Google Scholar]
- 63. Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves’ disease: a meta-analysis. Clin Endocrinol (Oxf). 2009;70(6):938–945. [DOI] [PubMed] [Google Scholar]
- 64. Collins JE, Heward JM, Nithiyananthan R, Nejentsev S, Todd JA, Franklyn JA, Gough SC. Lack of association of the vitamin D receptor gene with Graves’ disease in UK Caucasians. Clin Endocrinol (Oxf). 2004;60(5):618–624. [DOI] [PubMed] [Google Scholar]
- 65. Ramos-Lopez E, Kurylowicz A, Bednarczuk T, Paunkovic J, Seidl C, Badenhoop K. Vitamin D receptor polymorphisms are associated with Graves’ disease in German and Polish but not in Serbian patients. Thyroid. 2005;15(10):1125–1130. [DOI] [PubMed] [Google Scholar]
- 66. Pani MA, Regulla K, Segni M, Hofmann S, Hüfner M, Pasquino AM, Usadel KH, Badenhoop K. A polymorphism within the vitamin D-binding protein gene is associated with Graves’ disease but not with Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2002;87(6):2564–2567. [DOI] [PubMed] [Google Scholar]
- 67. Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342(8867):337–338. [DOI] [PubMed] [Google Scholar]
- 68. Agretti P, Chiovato L, De Marco G, Marcocci C, Mazzi B, Sellari-Franceschini S, Vitti P, Pinchera A, Tonacchera M. Real-time PCR provides evidence for thyrotropin receptor mRNA expression in orbital as well as in extraorbital tissues. Eur J Endocrinol. 2002;147(6):733–739. [DOI] [PubMed] [Google Scholar]
- 69. Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Núñez Miguel R, Blundell TL, Furmaniak J, Rees Smith B. Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid. 2004;14(8):560–570. [DOI] [PubMed] [Google Scholar]
- 70. Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3(4):297–300. [DOI] [PubMed] [Google Scholar]
- 71. Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010;20(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013;8(9):e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smith TJ. Fibroblast biology in thyroid diseases. Curr Opin Endocrinol Diabetes Obes. 2002;9(5):393–400. [Google Scholar]
- 74. Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87(1):385–392. [DOI] [PubMed] [Google Scholar]
- 75. Wang HS, Cao HJ, Winn VD, Rezanka LJ, Frobert Y, Evans CH, Sciaky D, Young DA, Smith TJ. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996;271(37):22718–22728. [PubMed] [Google Scholar]
- 76. Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine-target cell interactions. Proc Natl Acad Sci USA. 1998;95(15):8904–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smith TJ, Wang HS, Hogg MG, Henrikson RC, Keese CR, Giaever I. Prostaglandin E2 elicits a morphological change in cultured orbital fibroblasts from patients with Graves ophthalmopathy. Proc Natl Acad Sci USA. 1994;91(11):5094–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268(2 Pt 1):C382–C388. [DOI] [PubMed] [Google Scholar]
- 79. Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999;277(6 Pt 1):C1075–C1085. [DOI] [PubMed] [Google Scholar]
- 80. Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81(9):3428–3431. [DOI] [PubMed] [Google Scholar]
- 81. Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32(2):477–485. [DOI] [PubMed] [Google Scholar]
- 83. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355–16364. [DOI] [PubMed] [Google Scholar]
- 84. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615–29625. [DOI] [PubMed] [Google Scholar]
- 85. Roper RL, Conrad DH, Brown DM, Warner GL, Phipps RP. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J Immunol. 1990;145(8):2644–2651. [PubMed] [Google Scholar]
- 86. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146(1):108–113. [PubMed] [Google Scholar]
- 87. Tao W, Ayala-Haedo JA, Field MG, Pelaez D, Wester ST. RNA-sequencing gene expression profiling of orbital adipose-derived stem cell population implicate HOX genes and WNT signaling dysregulation in the pathogenesis of thyroid-associated orbitopathy. Invest Ophthalmol Vis Sci. 2017;58(14):6146–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fang S, Huang Y, Wang S, Zhang Y, Luo X, Liu L, Zhong S, Liu X, Li D, Liang R, Miranda P, Gu P, Zhou H, Fan X, Li B. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibroblasts in TAO. J Clin Endocrinol Metab. 2016;101(8):2955–2965. [DOI] [PubMed] [Google Scholar]
- 89. Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):197–203. [DOI] [PubMed] [Google Scholar]
- 90. van Steensel L, Paridaens D, Dingjan GM, van Daele PL, van Hagen PM, Kuijpers RW, van den Bosch WA, Drexhage HA, Hooijkaas H, Dik WA. Platelet-derived growth factor-BB: a stimulus for cytokine production by orbital fibroblasts in Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2010;51(2):1002–1007. [DOI] [PubMed] [Google Scholar]
- 91. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1beta in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84(11):4079–4084. [DOI] [PubMed] [Google Scholar]
- 92. Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273(39):25117–25124. [DOI] [PubMed] [Google Scholar]
- 93. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286(27):24487–24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grieb G, Bucala R. Fibrocytes in fibrotic diseases and wound healing. Adv Wound Care (New Rochelle). 2012;1(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Galligan CL, Keystone EC, Fish EN. Fibrocyte and T cell interactions promote disease pathogenesis in rheumatoid arthritis. J Autoimmun. 2016;69:38–50. [DOI] [PubMed] [Google Scholar]
- 96. Husseini M, Wang GS, Patrick C, Crookshank JA, MacFarlane AJ, Noel JA, Strom A, Scott FW. Heme oxygenase-1 induction prevents autoimmune diabetes in association with pancreatic recruitment of M2-like macrophages, mesenchymal cells, and fibrocytes. Endocrinology. 2015;156(11):3937–3949. [DOI] [PubMed] [Google Scholar]
- 97. Russell TM, Herzog EL, Bucala R. Flow cytometric identification of fibrocytes in scleroderma lung disease. Methods Mol Biol. 2012;900:327–346. [DOI] [PubMed] [Google Scholar]
- 98. Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94(12):6307–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Grab DJ, Lanners H, Martin LN, Chesney J, Cai C, Adkisson HD, Bucala R. Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol Med. 1999;5(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 102. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. [DOI] [PubMed] [Google Scholar]
- 103. Fernando R, Vonberg A, Atkins SJ, Pietropaolo S, Pietropaolo M, Smith TJ. Human fibrocytes express multiple antigens associated with autoimmune endocrine diseases. J Clin Endocrinol Metab. 2014;99(5):E796–E803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99(7):E1236–E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, Smith TJ. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109(19):7427–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fernando RGA, Lu Y, Atkins S, Smith TJ. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fiborcytes in Graves’ disease. J Immunol. 2018;200(12):3942–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93(6):2738–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. de Carli M, D’Elios MM, Mariotti S, Marcocci C, Pinchera A, Ricci M, Romagnani S, del Prete G. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77(5):1120–1124. [DOI] [PubMed] [Google Scholar]
- 109. Kahaly GJ, Shimony O, Gellman YN, Lytton SD, Eshkar-Sebban L, Rosenblum N, Refaeli E, Kassem S, Ilany J, Naor D. Regulatory T-cells in Graves’ orbitopathy: baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab. 2011;96(2):422–429. [DOI] [PubMed] [Google Scholar]
- 110. Jaume JC, Portolano S, Prummel MF, McLachlan SM, Rapoport B. Molecular cloning and characterization of genes for antibodies generated by orbital tissue-infiltrating B-cells in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1994;78(2):348–352. [DOI] [PubMed] [Google Scholar]
- 111. Gillespie EF, Raychaudhuri N, Papageorgiou KI, Atkins SJ, Lu Y, Charara LK, Mester T, Smith TJ, Douglas RS. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-κB. Invest Ophthalmol Vis Sci. 2012;53(12):7746–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mester T, Raychaudhuri N, Gillespie EF, Chen H, Smith TJ, Douglas RS. CD40 expression in fibrocytes is induced by TSH: potential synergistic immune activation. PLoS One. 2016;11(9):e0162994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK, Goncalves AC, Zhao SX, Ginter A, Lu Y, Smith TJ, Douglas RS. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97(5):E740–E746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yuan Q, Zhao Y, Zhu X, Liu X. Low regulatory T cell and high IL-17 mRNA expression in a mouse Graves’ disease model. J Endocrinol Invest. 2017;40(4):397–407. [DOI] [PubMed] [Google Scholar]
- 115. van Steensel L, Paridaens D, van Meurs M, van Hagen PM, van den Bosch WA, Kuijpers RW, Drexhage HA, Hooijkaas H, Dik WA. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2012;97(3):E400–E408. [DOI] [PubMed] [Google Scholar]
- 116. Virakul S, Phetsuksiri T, van Holten-Neelen C, Schrijver B, van Steensel L, Dalm VASH, Paridaens D, van den Bosch WA, van Hagen PM, Dik WA. Histamine induces NF-κB controlled cytokine secretion by orbital fibroblasts via histamine receptor type-1. Exp Eye Res. 2016;147:85–93. [DOI] [PubMed] [Google Scholar]
- 117. Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in coculture: evidence for up-regulation of prostaglandin E2 and hyaluronan synthesis. Endocrinology. 1999;140(8):3518–3525. [DOI] [PubMed] [Google Scholar]
- 118. Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10(3):366–391. [DOI] [PubMed] [Google Scholar]
- 119. Drexhage HA. Are there more than antibodies to the thyroid-stimulating hormone receptor that meet the eye in Graves’ disease? Endocrinology. 2006;147(1):9–12. [DOI] [PubMed] [Google Scholar]
- 120. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076–5080. [DOI] [PubMed] [Google Scholar]
- 121. Hopwood JJ, Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977;252(14):4777–4785. [PubMed] [Google Scholar]
- 122. Parmentier M, Libert F, Maenhaut C, Lefort A, Gérard C, Perret J, Van Sande J, Dumont JE, Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989;246(4937):1620–1622. [DOI] [PubMed] [Google Scholar]
- 123. Draman MS, Stechman M, Scott-Coombes D, Dayan CM, Rees DA, Ludgate M, Zhang L. The role of thyrotropin receptor activation in adipogenesis and modulation of fat phenotype. Front Endocrinol (Lausanne). 2017;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Endo T, Kobayashi T. Expression of functional TSH receptor in white adipose tissues of hyt/hyt mice induces lipolysis in vivo. Am J Physiol Endocrinol Metab. 2012;302(12):E1569–E1575. [DOI] [PubMed] [Google Scholar]
- 125. Santini F, Galli G, Maffei M, Fierabracci P, Pelosini C, Marsili A, Giannetti M, Castagna MG, Checchi S, Molinaro E, Piaggi P, Pacini F, Elisei R, Vitti P, Pinchera A. Acute exogenous TSH administration stimulates leptin secretion in vivo. Eur J Endocrinol. 2010;163(1):63–67. [DOI] [PubMed] [Google Scholar]
- 126. Shintani M, Nishimura H, Akamizu T, Yonemitsu S, Masuzaki H, Ogawa Y, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Thyrotropin decreases leptin production in rat adipocytes. Metabolism. 1999;48(12):1570–1574. [DOI] [PubMed] [Google Scholar]
- 127. Adams DD, Purves HD, Sirett NE. The response of hypophysectomized mice to injections of human serum containing long-acting thyroid stimulator. Endocrinology. 1961;68(1):154–155. [DOI] [PubMed] [Google Scholar]
- 128. Morshed SA, Ma R, Latif R, Davies TF. Biased signaling by thyroid-stimulating hormone receptor-specific antibodies determines thyrocyte survival in autoimmunity. Sci Signal. 2018;11(514): eaah4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tabasum A, Khan I, Taylor P, Das G, Okosieme OE. Thyroid antibody-negative euthyroid Graves' ophthalmopathy. Endocrinol Diabetes Metab Case Rep. 2016;2016:160008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jang SY, Shin DY, Lee EJ, Lee SY, Yoon JS. Relevance of TSH-receptor antibody levels in predicting disease course in Graves’ orbitopathy: comparison of the third-generation TBII assay and Mc4-TSI bioassay [published correction appears in Eye (Lond) 2013;27(10):1231]. Eye (Lond). 2013;27(8):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Teng CS, Smith BR, Anderson J, Hall R. Comparison of thyrotrophin receptors in membranes prepared from fat and thyroid tissue. Biochem Biophys Res Commun. 1975;66(2):836–841. [DOI] [PubMed] [Google Scholar]
- 132. Davies TF, Kimura H, Fong P, Kendler D, Shultz LD, Thung S, Martin A. The SCID-hu mouse and thyroid autoimmunity: characterization of human thyroid autoantibody secretion. Clin Immunol Immunopathol. 1991;60(2):319–330. [DOI] [PubMed] [Google Scholar]
- 133. Kikuoka S, Shimojo N, Yamaguchi KI, Watanabe Y, Hoshioka A, Hirai A, Saito Y, Tahara K, Kohn LD, Maruyama N, Kohno Y, Niimi H. The formation of thyrotropin receptor (TSHR) antibodies in a Graves’ animal model requires the N-terminal segment of the TSHR extracellular domain. Endocrinology. 1998;139(4):1891–1898. [DOI] [PubMed] [Google Scholar]
- 134. Costagliola S, Many MC, Denef JF, Pohlenz J, Refetoff S, Vassart G. Genetic immunization of outbred mice with thyrotropin receptor cDNA provides a model of Graves’ disease. J Clin Invest. 2000;105(6):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M. A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168(6):2789–2794. [DOI] [PubMed] [Google Scholar]
- 136. Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves’-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170(7):3522–3527. [DOI] [PubMed] [Google Scholar]
- 137. Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003;111(12):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim-Saijo M, Akamizu T, Ikuta K, Iida Y, Ohmori K, Matsubara K, Matsuda Y, Suzuki M, Matsuda F, Nakao K. Generation of a transgenic animal model of hyperthyroid Graves’ disease. Eur J Immunol. 2003;33(9):2531–2538. [DOI] [PubMed] [Google Scholar]
- 139. Kaneda T, Honda A, Hakozaki A, Fuse T, Muto A, Yoshida T. An improved Graves’ disease model established by using in vivo electroporation exhibited long-term immunity to hyperthyroidism in BALB/c mice. Endocrinology. 2007;148(5):2335–2344. [DOI] [PubMed] [Google Scholar]
- 140. Zhao SX, Tsui S, Cheung A, Douglas RS, Smith TJ, Banga JP. Orbital fibrosis in a mouse model of Graves’ disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011;210(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013;154(9):3008–3015. [DOI] [PubMed] [Google Scholar]
- 142. Berchner-Pfannschmidt U, Moshkelgosha S, Diaz-Cano S, Edelmann B, Görtz GE, Horstmann M, Noble A, Hansen W, Eckstein A, Banga JP. Comparative assessment of female mouse model of Graves’ orbitopathy under different environments, accompanied by proinflammatory cytokine and T-cell responses to thyrotropin hormone receptor antigen. Endocrinology. 2016;157(4):1673–1682. [DOI] [PubMed] [Google Scholar]
- 143. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program . Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases [published correction appears in Proc Natl Acad Sci USA 2015;112(10):E1163–E1167]. Proc Natl Acad Sci USA. 2015;112(4):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. LeRoith D, Werner H, Beitner-Johnson D, Roberts CT Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16(2):143–163. [DOI] [PubMed] [Google Scholar]
- 146. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. [DOI] [PubMed] [Google Scholar]
- 147. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787. [DOI] [PubMed] [Google Scholar]
- 148. De Meyts PSW, Palsgaard J, Theede A, Gaugain L, Aladdin H, Whittaker J. Insulin and IGF-I receptor structure and binding mechanism In: Saltiel AREPJ, ed. Mechanisms of Insulin Action. Austin, TX: Landes Bioscience; 2007:1–32. [Google Scholar]
- 149. Varewijck AJ, Janssen JA. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer. 2012;19(5):F63–F75. [DOI] [PubMed] [Google Scholar]
- 150. Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5(10):2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kavran JMMJ, McCabe JM, Byrne PO, Connacher MK, Wang Z, Ramek A, Sarabipour S, Shan Y, Shaw DE, Hristova K, Cole PA, Leahy DJ. How IGF-1 activates its receptor. eLife. 2014;3:e03772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Boucher J, Charalambous M, Zarse K, Mori MA, Kleinridders A, Ristow M, Ferguson-Smith AC, Kahn CR. Insulin and insulin-like growth factor 1 receptors are required for normal expression of imprinted genes. Proc Natl Acad Sci USA. 2014;111(40):14512–14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lopaczynski W, Terry C, Nissley P. Autophosphorylation of the insulin-like growth factor I receptor cytoplasmic domain. Biochem Biophys Res Commun. 2000;279(3):955–960. [DOI] [PubMed] [Google Scholar]
- 154. Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277(42):39684–39695. [DOI] [PubMed] [Google Scholar]
- 155. De Meyts P, Wallach B, Christoffersen CT, Ursø B, Grønskov K, Latus LJ, Yakushiji F, Ilondo MM, Shymko RM. The insulin-like growth factor-I receptor. Structure, ligand-binding mechanism and signal transduction. Horm Res. 1994;42(4-5):152–169. [DOI] [PubMed] [Google Scholar]
- 156. Slaaby R, Schäffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. [DOI] [PubMed] [Google Scholar]
- 157. King ER, Wong KK. Insulin-like growth factor: current concepts and new developments in cancer therapy. Recent Patents Anticancer Drug Discov. 2012;7(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. LeRoith DAD, Werner H, Roberts C Jr. Molecular and cellular biology of the insulin-like growth factors In: Weintraub BD, ed. Molecular Endocrinology, Basic Concepts and Clinical Correlations. New York, NY: Raven Press; 1995:181–193. [Google Scholar]
- 159. Roberts CT., Jr Control of insulin-like growth factor (IGF) action by regulation of IGF-I receptor expression. Endocr J. 1996;43(Suppl):S49–S55. [DOI] [PubMed] [Google Scholar]
- 160. Rosenfeld RG, Roberts CT, Jr., eds. The IGF System: Molecular Biology, Physiology, and Clinical Applications. Totowa, NJ: Humana Press Inc.; 1999:63–88. [Google Scholar]
- 161. Yamamoto H, Prager D, Yamasaki H, Melmed S. Rat pituitary GC cell insulin-like growth factor-I receptor regulation. Endocrinology. 1993;133(3):1420–1425.7689956 [Google Scholar]
- 162. Ceda GP, Fielder PJ, Donovan SM, Rosenfeld RG, Hoffman AR. Regulation of insulin-like growth factor-binding protein expression by thyroid hormone in rat GH3 pituitary tumor cells. Endocrinology. 1992;130(3):1483–1489. [DOI] [PubMed] [Google Scholar]
- 163. Baserga R, Sell C, Porcu P, Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994;27(2):63–71. [DOI] [PubMed] [Google Scholar]
- 164. Rosenthal SM, Brown EJ, Brunetti A, Goldfine ID. Fibroblast growth factor inhibits insulin-like growth factor-II (IGF-II) gene expression and increases IGF-I receptor abundance in BC3H-1 muscle cells. Mol Endocrinol. 1991;5(5):678–684. [DOI] [PubMed] [Google Scholar]
- 165. DeAngelis T, Ferber A, Baserga R. Insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the platelet-derived growth factor receptor. J Cell Physiol. 1995;164(1):214–221. [DOI] [PubMed] [Google Scholar]
- 166. Coppola D, Ferber A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14(7):4588–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76(4):1005–1026. [DOI] [PubMed] [Google Scholar]
- 168. Salmon WD Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49(6):825–836. [PubMed] [Google Scholar]
- 169. Salmon WD Jr, DuVall MR. A serum fraction with “sulfation factor activity” stimulates in vitro incorporation of leucine and sulfate into protein-polysaccharide complexes, uridine into RNA, and thymidine into DNA of costal cartilage from hypophysectomized rats. Endocrinology. 1970;86(4):721–727. [DOI] [PubMed] [Google Scholar]
- 170. Daughaday WH, Reeder C. Synchronous activation of DNA synthesis in hypophysectomized rat cartilage by growth hormone. J Lab Clin Med. 1966;68(3):357–368. [PubMed] [Google Scholar]
- 171. Daughaday WH, Mariz IK. Conversion of proline U-C14 to labeled hydroxyproline by rat cartilage in vitro: effects of hypophysectomy, growth hormone, and cortisol. J Lab Clin Med. 1962;59:741–752. [PubMed] [Google Scholar]
- 172. Daughaday WH, Hall K, Raben MS, Salmon WD Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature. 1972;235(5333):107. [DOI] [PubMed] [Google Scholar]
- 173. Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978;89(2):283–286. [DOI] [PubMed] [Google Scholar]
- 174. Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253(8):2769–2776. [PubMed] [Google Scholar]
- 175. Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336(9):633–640. [DOI] [PubMed] [Google Scholar]
- 176. Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107(6):873–877. [DOI] [PubMed] [Google Scholar]
- 177. Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19(49):5574–5581. [DOI] [PubMed] [Google Scholar]
- 178. Petley T, Graff K, Jiang W, Yang H, Florini J. Variation among cell types in the signaling pathways by which IGF-I stimulates specific cellular responses. Horm Metab Res. 1999;31(2-3):70–76. [DOI] [PubMed] [Google Scholar]
- 179. Holly J, Sabin M, Perks C, Shield J. Adipogenesis and IGF-1. Metab Syndr Relat Disord. 2006;4(1):43–50. [DOI] [PubMed] [Google Scholar]
- 180. Villafuerte BC, Fine JB, Bai Y, Zhao W, Fleming S, DiGirolamo M. Expressions of leptin and insulin-like growth factor-I are highly correlated and region-specific in adipose tissue of growing rats. Obes Res. 2000;8(9):646–655. [DOI] [PubMed] [Google Scholar]
- 181. Zizola CF, Balañá ME, Sandoval M, Calvo JC. Changes in IGF-I receptor and IGF-I mRNA during differentiation of 3T3-L1 preadipocytes. Biochimie. 2002;84(10):975–980. [DOI] [PubMed] [Google Scholar]
- 182. Modan-Moses D, Janicot M, McLenithan JC, Lane MD, Casella SJ. Expression and function of insulin/insulin-like growth factor I hybrid receptors during differentiation of 3T3-L1 preadipocytes. Biochem J. 1998;333(Pt 3):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gross SM, Rotwein P. Unraveling growth factor signaling and cell cycle progression in individual fibroblasts. J Biol Chem. 2016;291(28):14628–14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Brandt K, Grünler J, Brismar K, Wang J. Effects of IGFBP-1 and IGFBP-2 and their fragments on migration and IGF-induced proliferation of human dermal fibroblasts. Growth Horm IGF Res. 2015;25(1):34–40. [DOI] [PubMed] [Google Scholar]
- 185. Zhao P, Deng Y, Gu P, Wang Y, Zhou H, Hu Y, Chen P, Fan X. Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy. Exp Eye Res. 2013;107:65–73. [DOI] [PubMed] [Google Scholar]
- 186. Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab. 1995;80(2):637–647. [DOI] [PubMed] [Google Scholar]
- 187. Clemmons DR. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J Mol Endocrinol. 2018;61(1):T139–T169. [DOI] [PubMed] [Google Scholar]
- 188. Janssen JA, van der Lely AJ, Lamberts SW. Circulating free insulin-like growth-factor-I (IGF-I) levels should also be measured to estimate the IGF-I bioactivity. J Endocrinol Invest. 2003;26(6):588–594. [DOI] [PubMed] [Google Scholar]
- 189. Varewijck AJ, Boelen A, Lamberts SW, Fliers E, Hofland LJ, Wiersinga WM, Janssen JA. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013;98(2):769–776. [DOI] [PubMed] [Google Scholar]
- 190. Worrall C, Nedelcu D, Serly J, Suleymanova N, Oprea I, Girnita A, Girnita L. Novel mechanisms of regulation of IGF-1R action: functional and therapeutic implications. Pediatr Endocrinol Rev. 2013;10(4):473–484. [PubMed] [Google Scholar]
- 191. Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am. 2012;26(3):527–542, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17–S27. [DOI] [PubMed] [Google Scholar]
- 193. Myers MG Jr, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3′-kinase. Endocrinology. 1993;132(4):1421–1430. [DOI] [PubMed] [Google Scholar]
- 194. Myers MG Jr, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42(5):643–650. [DOI] [PubMed] [Google Scholar]
- 195. Clemmons D. Insulin-like growth factor-1 and its binding proteins In: Jameson JL, De Groot LJ, eds. Endocrinology: Adult and Pediatric. 7th ed.Philadelphia, PA: Elsevier Saunders; 2016:359–381. [Google Scholar]
- 196. Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci. 2014;71(13):2403–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Crudden C, Girnita A, Girnita L. Targeting the IGF-1R: the tale of the tortoise and the hare. Front Endocrinol (Lausanne). 2015;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Dalle S, Imamura T, Rose DW, Worrall DS, Ugi S, Hupfeld CJ, Olefsky JM. Insulin induces heterologous desensitization of G-protein-coupled receptor and insulin-like growth factor I signaling by downregulating beta-arrestin-1. Mol Cell Biol. 2002;22(17):6272–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Reiter E, Ayoub MA, Pellissier LP, Landomiel F, Musnier A, Tréfier A, Gandia J, De Pascali F, Tahir S, Yvinec R, Bruneau G, Poupon A, Crépieux P. β-Arrestin signalling and bias in hormone-responsive GPCRs. Mol Cell Endocrinol. 2017;449:28–41. [DOI] [PubMed] [Google Scholar]
- 200. Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12(3):205–216. [DOI] [PubMed] [Google Scholar]
- 201. Suleymanova N, Crudden C, Worrall C, Dricu A, Girnita A, Girnita L. Enhanced response of melanoma cells to MEK inhibitors following unbiased IGF-1R down-regulation. Oncotarget. 2017;8(47):82256–82267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Girnita A, Zheng H, Grönberg A, Girnita L, Ståhle M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31(3):352–365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 203. Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92(5):399–408. [DOI] [PubMed] [Google Scholar]
- 204. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–1160. [DOI] [PubMed] [Google Scholar]
- 205. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Irannejad R, Tsvetanova NG, Lobingier BT, von Zastrow M. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol. 2015;35:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Girnita L, Takahashi SI, Crudden C, Fukushima T, Worrall C, Furuta H, Yoshihara H, Hakuno F, Girnita A. Chapter seven—when phosphorylation encounters ubiquitination: a balanced perspective on IGF-1R signaling. Prog Mol Biol Transl Sci. 2016;141:277–311. [DOI] [PubMed] [Google Scholar]
- 208. Sarfstein R, Werner H. Minireview: nuclear insulin and insulin-like growth factor-1 receptors: a novel paradigm in signal transduction. Endocrinology. 2013;154(5):1672–1679. [DOI] [PubMed] [Google Scholar]
- 209. Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70(16):6412–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hoa N, Tsui S, Afifiyan NF, Sinha Hikim A, Li B, Douglas RS, Smith TJ. Nuclear targeting of IGF-1 receptor in orbital fibroblasts from Graves’ disease: apparent role of ADAM17. PLoS One. 2012;7(4):e34173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vieira M, Gomes JR, Saraiva MJ. Transthyretin induces insulin-like growth factor I nuclear translocation regulating its levels in the hippocampus. Mol Neurobiol. 2015;51(3):1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Buxbaum JN, Reixach N. Transthyretin: the servant of many masters. Cell Mol Life Sci. 2009;66(19):3095–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Abboud SL, Bethel CR, Aron DC. Secretion of insulin-like growth factor I and insulin-like growth factor-binding proteins by murine bone marrow stromal cells. J Clin Invest. 1991;88(2):470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Weigent DA. Lymphocyte GH-axis hormones in immunity. Cell Immunol. 2013;285(1-2):118–132. [DOI] [PubMed] [Google Scholar]
- 215. Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999;10(1):5–14. [DOI] [PubMed] [Google Scholar]
- 216. Jardieu P, Clark R, Mortensen D, Dorshkind K. In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. J Immunol. 1994;152(9):4320–4327. [PubMed] [Google Scholar]
- 217. Clark R, Strasser J, McCabe S, Robbins K, Jardieu P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest. 1993;92(2):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kimata H, Yoshida A. Effect of growth hormone and insulin-like growth factor-I on immunoglobulin production by and growth of human B cells. J Clin Endocrinol Metab. 1994;78(3):635–641. [DOI] [PubMed] [Google Scholar]
- 219. Zhao X, McBride BW, Trouten-Radford LM, Burton JH. Specific insulin-like growth factor-I receptors on circulating bovine mononuclear cells. J Recept Res. 1992;12(1):117–129. [DOI] [PubMed] [Google Scholar]
- 220. Kooijman R, Willems M, De Haas CJ, Rijkers GT, Schuurmans AL, Van Buul-Offers SC, Heijnen CJ, Zegers BJ. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992;131(5):2244–2250. [DOI] [PubMed] [Google Scholar]
- 221. Janssen JA, Hoogerbrugge N, van Neck JW, Uitterlinden P, Lamberts SW. The IGF-I/IGFBP system in congenital partial lipodystrophy. Clin Endocrinol (Oxf). 1998;49(4):465–473. [DOI] [PubMed] [Google Scholar]
- 222. Kooijman R, Scholtens LE, Rijkers GT, Zegers BJ. Type I insulin-like growth factor receptor expression in different developmental stages of human thymocytes. J Endocrinol. 1995;147(2):203–209. [DOI] [PubMed] [Google Scholar]
- 223. Kooijman R, van Buul-Offers SC, Scholtens LE, Schuurman HJ, Van den Brande LJ, Zegers BJ. T cell development in insulin-like growth factor-II transgenic mice. J Immunol. 1995;154(11):5736–5745. [PubMed] [Google Scholar]
- 224. Kooijman RK, Scholtens LE, Rijkers GT, Zegers BJ. Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Eur J Immunol. 1995;25(4):931–935. [DOI] [PubMed] [Google Scholar]
- 225. Schillaci R, Brocardo MG, Galeano A, Roldán A. Downregulation of insulin-like growth factor-1 receptor (IGF-1R) expression in human T lymphocyte activation. Cell Immunol. 1998;183(2):157–161. [DOI] [PubMed] [Google Scholar]
- 226. Stuart CA, Meehan RT, Neale LS, Cintron NM, Furlanetto RW. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991;72(5):1117–1122. [DOI] [PubMed] [Google Scholar]
- 227. Verland S, Gammeltoft S. Functional receptors for insulin-like growth factors I and II in rat thymocytes and mouse thymoma cells. Mol Cell Endocrinol. 1989;67(2-3):207–216. [DOI] [PubMed] [Google Scholar]
- 228. Kooijman R, Coppens A, Hooghe-Peters E. Igf-I inhibits spontaneous apoptosis in human granulocytes. Endocrinology. 2002;143(4):1206–1212. [DOI] [PubMed] [Google Scholar]
- 229. Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7(11):2243–2248. [PubMed] [Google Scholar]
- 230. Tapson VF, Boni-Schnetzler M, Pilch PF, Center DM, Berman JS. Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor I in vitro. J Clin Invest. 1988;82(3):950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Johnson EW, Jones LA, Kozak RW. Expression and function of insulin-like growth factor receptors on anti-CD3-activated human T lymphocytes. J Immunol. 1992;148(1):63–71. [PubMed] [Google Scholar]
- 232. Xu X, Mardell C, Xian CJ, Zola H, Read LC. Expression of functional insulin-like growth factor-1 receptor on lymphoid cell subsets of rats. Immunology. 1995;85(3):394–399. [PMC free article] [PubMed] [Google Scholar]
- 233. Gjerset RA, Yeargin J, Volkman SK, Vila V, Arya J, Haas M. Insulin-like growth factor-I supports proliferation of autocrine thymic lymphoma cells with a pre-T cell phenotype. J Immunol. 1990;145(10):3497–3501. [PubMed] [Google Scholar]
- 234. Funk PE, Kincade PW, Witte PL. Native associations of early hematopoietic stem cells and stromal cells isolated in bone marrow cell aggregates. Blood. 1994;83(2):361–369. [PubMed] [Google Scholar]
- 235. Clark R. The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev. 1997;18(2):157–179. [DOI] [PubMed] [Google Scholar]
- 236. Gibson LF, Piktel D, Landreth KS. Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood. 1993;82(10):3005–3011. [PubMed] [Google Scholar]
- 237. Landreth KS, Narayanan R, Dorshkind K. Insulin-like growth factor-I regulates pro-B cell differentiation. Blood. 1992;80(5):1207–1212. [PubMed] [Google Scholar]
- 238. Rom WN, Pääkkö P. Activated alveolar macrophages express the insulin-like growth factor-I receptor. Am J Respir Cell Mol Biol. 1991;4(5):432–439. [DOI] [PubMed] [Google Scholar]
- 239. Kooijman R, Coppens A, Hooghe-Peters E. IGF-I stimulates IL-8 production in the promyelocytic cell line HL-60 through activation of extracellular signal-regulated protein kinase. Cell Signal. 2003;15(12):1091–1098. [DOI] [PubMed] [Google Scholar]
- 240. Kooijman R, Rijkers GT, Zegers BJ. IGF-I potentiates interleukin-2 production in human peripheral T cells. J Endocrinol. 1996;149(2):351–356. [DOI] [PubMed] [Google Scholar]
- 241. Kooijman R, Coppens A. Insulin-like growth factor-I stimulates IL-10 production in human T cells. J Leukoc Biol. 2004;76(4):862–867. [DOI] [PubMed] [Google Scholar]
- 242. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178(5):3281–3287. [DOI] [PubMed] [Google Scholar]
- 243. Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. B Cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181(8):5768–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348–6354. [DOI] [PubMed] [Google Scholar]
- 245. Douglas RS, Brix TH, Hwang CJ, Hegedüs L, Smith TJ. Divergent frequencies of IGF-I receptor-expressing blood lymphocytes in monozygotic twin pairs discordant for Graves’ disease: evidence for a phenotypic signature ascribable to nongenetic factors. J Clin Endocrinol Metab. 2009;94(5):1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Nyman T, Pekonen F. The expression of insulin-like growth factors and their binding proteins in normal human lymphocytes. Acta Endocrinol (Copenh). 1993;128(2):168–172. [DOI] [PubMed] [Google Scholar]
- 247. Auernhammer CJ, Fottner C, Engelhardt D, Bidlingmaier M, Strasburger CJ, Weber MM. Differential regulation of insulin-like growth factor-(IGF) I and IGF-binding protein (IGFBP) secretion by human peripheral blood mononuclear cells. Horm Res. 2002;57(1-2):15–21. [DOI] [PubMed] [Google Scholar]
- 248. Rodriguez-Arnao J, Miell JP, Ross RJ. Influence of thyroid hormones on the GH-IGF-I axis. Trends Endocrinol Metab. 1993;4(5):169–173. [DOI] [PubMed] [Google Scholar]
- 249. Chernausek SD, Underwood LE, Utiger RD, Van Wyk JJ. Growth hormone secretion and plasma somatomedin-C in primary hypothyroidism. Clin Endocrinol (Oxf). 1983;19(3):337–344. [DOI] [PubMed] [Google Scholar]
- 250. Wolf M, Ingbar SH, Moses AC. Thyroid hormone and growth hormone interact to regulate insulin-like growth factor-I messenger ribonucleic acid and circulating levels in the rat. Endocrinology. 1989;125(6):2905–2914. [DOI] [PubMed] [Google Scholar]
- 251. Iranmanesh A, Lizarralde G, Johnson ML, Veldhuis JD. Nature of altered growth hormone secretion in hyperthyroidism. J Clin Endocrinol Metab. 1991;72(1):108–115. [DOI] [PubMed] [Google Scholar]
- 252. Fagin JA, Fernandez-Mejia C, Melmed S. Pituitary insulin-like growth factor-I gene expression: regulation by triiodothyronine and growth hormone. Endocrinology. 1989;125(5):2385–2391. [DOI] [PubMed] [Google Scholar]
- 253. Matsuo K, Yamashita S, Niwa M, Kurihara M, Harakawa S, Izumi M, Nagataki S, Melmed S. Thyroid hormone regulates rat pituitary insulin-like growth factor-I receptors. Endocrinology. 1990;126(1):550–554. [DOI] [PubMed] [Google Scholar]
- 254. Jorgensen JOWT, Weeke J. Growth hormone and thyroid function and energy expenditue In: Juul AJJ, ed. Growth Hormone in Adults: Physiological and Clinical Aspects. Cambridge, UK: Cambridge University Press; 2000:333–348. [Google Scholar]
- 255. Root AW, Snyder PJ, Rezvani I, DiGeorge AM, Utiger RD. Inhibition of thyrotropin releasing hormone-mediated secretion of thyrotropin by human growth hormone. J Clin Endocrinol Metab. 1973;36(1):103–107. [DOI] [PubMed] [Google Scholar]
- 256. Lippe BM, Van Herle AJ, LaFranchi SH, Uller RP, Lavin N, Kaplan SA. Reversible hypothyroidism in growth hormone-deficient children treated with human growth hormone. J Clin Endocrinol Metab. 1975;40(4):612–618. [DOI] [PubMed] [Google Scholar]
- 257. Jørgensen JO, Møller J, Laursen T, Orskov H, Christiansen JS, Weeke J. Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: studies in GH-deficient adults. Clin Endocrinol (Oxf). 1994;41(5):609–614. [DOI] [PubMed] [Google Scholar]
- 258. Jørgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab. 1989;69(6):1127–1132. [DOI] [PubMed] [Google Scholar]
- 259. Grunfeld C, Sherman BM, Cavalieri RR. The acute effects of human growth hormone administration on thyroid function in normal men. J Clin Endocrinol Metab. 1988;67(5):1111–1114. [DOI] [PubMed] [Google Scholar]
- 260. Gaspard T, Wondergem R, Hamamdzić M, Klitgaard HM. Serum somatomedin stimulation in thyroxine-treated hypophysectomized rats. Endocrinology. 1978;102(2):606–611. [DOI] [PubMed] [Google Scholar]
- 261. Kupfer JM, Rubin SA. Differential regulation of insulin-like growth factor I by growth hormone and thyroid hormone in the heart of juvenile hypophysectomized rats. J Mol Cell Cardiol. 1992;24(6):631–639. [DOI] [PubMed] [Google Scholar]
- 262. Sabatino L, Gliozheni E, Molinaro S, Bonotti A, Azzolina S, Popoff G, Carpi A, Iervasi G. Thyroid hormone receptor and IGF1/IGFR systems: possible relations in the human heart. Biomed Pharmacother. 2007;61(8):457–462. [DOI] [PubMed] [Google Scholar]
- 263. Ramajayam G, Vignesh RC, Karthikeyan S, Kumar KS, Karthikeyan GD, Veni S, Sridhar M, Arunakaran J, Aruldhas MM, Srinivasan N. Regulation of insulin-like growth factors and their binding proteins by thyroid stimulating hormone in human osteoblast-like (SaOS2) cells. Mol Cell Biochem. 2012;368(1-2):77–88. [DOI] [PubMed] [Google Scholar]
- 264. Snyder P. The pituitary in hypothyroidism In: Lebrd U, ed. The Thyroid. 7th ed.Piladelphia, PA: Lippincott-Raven Publishers; 1996:836–844. [Google Scholar]
- 265. Chernausek SD, Turner R. Attenuation of spontaneous, nocturnal growth hormone secretion in children with hypothyroidism and its correlation with plasma insulin-like growth factor I concentrations. J Pediatr. 1989;114(6):968–972. [DOI] [PubMed] [Google Scholar]
- 266. Miell JP, Taylor AM, Zini M, Maheshwari HG, Ross RJ, Valcavi R. Effects of hypothyroidism and hyperthyroidism on insulin-like growth factors (IGFs) and growth hormone- and IGF-binding proteins. J Clin Endocrinol Metab. 1993;76(4):950–955. [DOI] [PubMed] [Google Scholar]
- 267. Ramos-Dias JC, Yateman M, Camacho-Hübner C, Grossman A, Lengyel AM. Low circulating IGF-I levels in hyperthyroidism are associated with decreased GH response to GH-releasing hormone. Clin Endocrinol (Oxf). 1995;43(5):583–589. [DOI] [PubMed] [Google Scholar]
- 268. Välimäki M, Liewendahl, Karonen SL, Helenius T, Suikkari AM. Concentrations of somatomedin-C and triiodothyronine in patients with thyroid dysfunction and nonthyroidal illnesses. J Endocrinol Invest. 1990;13(2):155–159. [DOI] [PubMed] [Google Scholar]
- 269. Valcavi R, Zini M, Portioli I. Thyroid hormones and growth hormone secretion. J Endocrinol Invest. 1992;15(4):313–330. [DOI] [PubMed] [Google Scholar]
- 270. Thomas MR, Miell JP, Taylor AM, Ross RJ, Arnao JR, Jewitt DE, McGregor AM. Endocrine and cardiac paracrine actions of insulin-like growth factor-I (IGF-I) during thyroid dysfunction in the rat: is IGF-I implicated in the mechanism of heart weight/body weight change during abnormal thyroid function? J Mol Endocrinol. 1993;10(3):313–323. [DOI] [PubMed] [Google Scholar]
- 271. Rodriguez-Arnao J, Miell J, Thomas M, McGregor AM, Ross RJ. Changes in hepatic insulin-like growth factor-binding proteins -1, -2 and -3 mRNA levels in rats with altered thyroid status. J Endocrinol. 1994;140(2):251–255. [DOI] [PubMed] [Google Scholar]
- 272. Angervo M, Leinonen P, Koistinen R, Julkunen M, Seppälä M. Tri-iodothyronine and cycloheximide enhance insulin-like growth factor-binding protein-1 gene expression in human hepatoma cells. J Mol Endocrinol. 1993;10(1):7–13. [DOI] [PubMed] [Google Scholar]
- 273. Iglesias P, Bayón C, Méndez J, Gancedo PG, Grande C, Diez JJ. Serum insulin-like growth factor type 1, insulin-like growth factor-binding protein-1, and insulin-like growth factor-binding protein-3 concentrations in patients with thyroid dysfunction. Thyroid. 2001;11(11):1043–1048. [DOI] [PubMed] [Google Scholar]
- 274. Jenkins RC, Valcavi R, Zini M, Frasoldati A, Heller SR, Camacho-Hubner C, Gibson JM, Westwood M, Ross RJ. Association of elevated insulin-like growth factor binding protein-1 with insulin resistance in hyperthyroidism. Clin Endocrinol (Oxf). 2000;52(2):187–195. [DOI] [PubMed] [Google Scholar]
- 275. Frystyk J, Grønbaek H, Skjaerbaek C, Flyvbjerg A. Effect of hyperthyroidism on circulating levels of free and total IGF-I and IGFBPs in rats. Am J Physiol. 1995;269(5 Pt 1):E840–E845. [DOI] [PubMed] [Google Scholar]
- 276. Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216(3):319–357. [DOI] [PubMed] [Google Scholar]
- 277. Zimmermann-Belsing T, Juul A, Juul Holst J, Feldt-Rasmussen U. The insulin-like growth axis in patients with autoimmune thyrotoxicosis: effect of antithyroid drug treatment. Growth Horm IGF Res. 2004;14(3):235–244. [DOI] [PubMed] [Google Scholar]
- 278. Janssen JA. Insulin-like growth factor I: pros and cons of a bioassay. Horm Res Paediatr. 2011;76(s1, Suppl 1):106–110. [DOI] [PubMed] [Google Scholar]
- 279. Lazarus NR. Insulin and insulin-like substances in the circulation. Clin Endocrinol Metab. 1972;1(3):623–644. [DOI] [PubMed] [Google Scholar]
- 280. Brugts MP, Ranke MB, Hofland LJ, van der Wansem K, Weber K, Frystyk J, Lamberts SW, Janssen JA. Normal values of circulating insulin-like growth factor-I bioactivity in the healthy population: comparison with five widely used IGF-I immunoassays. J Clin Endocrinol Metab. 2008;93(7):2539–2545. [DOI] [PubMed] [Google Scholar]
- 281. Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119(2):940–942. [DOI] [PubMed] [Google Scholar]
- 282. Vitti P, Rotella CM, Valente WA, Cohen J, Aloj SM, Laccetti P, Ambesi-Impiombato FS, Grollman EF, Pinchera A, Toccafondi R, Kohn LD. Characterization of the optimal stimulatory effects of graves’ monoclonal and serum immunoglobulin G on adenosine 3′,5′-monophosphate production in fRTL-5 thyroid cells: a potential clinical assay. J Clin Endocrinol Metab. 1983;57(4):782–791. [DOI] [PubMed] [Google Scholar]
- 283. Brenner-Gati L, Berg KA, Gershengorn MC. Thyroid-stimulating hormone and insulin-like growth factor-1 synergize to elevate 1,2-diacylglycerol in rat thyroid cells. Stimulation of DNA synthesis via interaction between lipid and adenylyl cyclase signal transduction systems. J Clin Invest. 1988;82(3):1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. J Clin Endocrinol Metab. 2015;100(3):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Clément S, Refetoff S, Robaye B, Dumont JE, Schurmans S. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142(12):5131–5139. [DOI] [PubMed] [Google Scholar]
- 286. Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Ock S, Ahn J, Lee SH, Kang H, Offermanns S, Ahn HY, Jo YS, Shong M, Cho BY, Jo D, Abel ED, Lee TJ, Park WJ, Lee IK, Kim J. IGF-1 receptor deficiency in thyrocytes impairs thyroid hormone secretion and completely inhibits TSH-stimulated goiter. FASEB J. 2013;27(12):4899–4908. [DOI] [PubMed] [Google Scholar]
- 288. Müller K, Führer D, Mittag J, Klöting N, Blüher M, Weiss RE, Many MC, Schmid KW, Krohn K. TSH compensates thyroid-specific IGF-I receptor knockout and causes papillary thyroid hyperplasia. Mol Endocrinol. 2011;25(11):1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Sastre-Perona A, Santisteban P. Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation. Mol Endocrinol. 2014;28(5):681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Bhaskar V, Goldfine ID, Bedinger DH, Lau A, Kuan HF, Gross LM, Handa M, Maddux BA, Watson SR, Zhu S, Narasimha AJ, Levy R, Webster L, Wijesuriya SD, Liu N, Wu X, Chemla-Vogel D, Tran C, Lee SR, Wong S, Wilcock D, White ML, Corbin JA. A fully human, allosteric monoclonal antibody that activates the insulin receptor and improves glycemic control. Diabetes. 2012;61(5):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh). 1989;121(6):753–758. [DOI] [PubMed] [Google Scholar]
- 292. O’Brien RM, Soos MA, Siddle K. Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J. 1987;6(13):4003–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Zick Y, Rees-Jones RW, Taylor SI, Gorden P, Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984;259(7):4396–4400. [PubMed] [Google Scholar]
- 294. Cazorla M, Arrang JM, Prémont J. Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor. Br J Pharmacol. 2011;162(4):947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Corbin JA, Bhaskar V, Goldfine ID, Bedinger DH, Lau A, Michelson K, Gross LM, Maddux BA, Kuan HF, Tran C, Lao L, Handa M, Watson SR, Narasimha AJ, Zhu S, Levy R, Webster L, Wijesuriya SD, Liu N, Wu X, Chemla-Vogel D, Lee SR, Wong S, Wilcock D, White ML. Improved glucose metabolism in vitro and in vivo by an allosteric monoclonal antibody that increases insulin receptor binding affinity. PLoS One. 2014;9(2):e88684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16(4):251–257. [DOI] [PubMed] [Google Scholar]
- 297. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942–950. [DOI] [PubMed] [Google Scholar]
- 298. Gianoukakis AG, Douglas RS, King CS, Cruikshank WW, Smith TJ. Immunoglobulin G from patients with Graves’ disease induces interleukin-16 and RANTES expression in cultured human thyrocytes: a putative mechanism for T-cell infiltration of the thyroid in autoimmune disease. Endocrinology. 2006;147(4):1941–1949. [DOI] [PubMed] [Google Scholar]
- 299. Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Köhrle J, Eckstein A, Schomburg L. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(2):752–760. [DOI] [PubMed] [Google Scholar]
- 300. Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. TSH/IGF-1 receptor cross talk in Graves’ ophthalmopathy pathogenesis. J Clin Endocrinol Metab. 2016;101(6):2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Smith TJ, Janssen JAMJL. Response to Krieger et al. re: “TSHR/IGF-1R cross-talk, not IGF-1R stimulating antibodies, mediates Graves’ ophthalmopathy pathogenesis” (Thyroid 2017;27:746–747). Thyroid. 2017;27(11):1458–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Kohn LD, Alvarez F, Marcocci C, Kohn AD, Corda D, Hoffman WE, Tombaccini D, Valente WA, de Luca M, Santisteban P, Grollman EF. Monoclonal antibody studies defining the origin and properties of autoantibodies in Graves’ disease. Ann N Y Acad Sci. 1986;475(1 Autoimmunity):157–173. [DOI] [PubMed] [Google Scholar]
- 303. Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28(12):602–607. [DOI] [PubMed] [Google Scholar]
- 304. Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32(8):443–450. [DOI] [PubMed] [Google Scholar]
- 305. Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270(28):16495–16498. [DOI] [PubMed] [Google Scholar]
- 306. Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276(19):15688–15695. [DOI] [PubMed] [Google Scholar]
- 307. Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278(51):51334–51339. [DOI] [PubMed] [Google Scholar]
- 308. Lin FT, Daaka Y, Lefkowitz RJ. beta-Arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem. 1998;273(48):31640–31643. [DOI] [PubMed] [Google Scholar]
- 309. Nagayama Y, Takeshita A, Luo W, Ashizawa K, Yokoyama N, Nagataki S. High affinity binding of thyrotropin (TSH) and thyroid-stimulating autoantibody for the TSH receptor extracellular domain. Thyroid. 1994;4(2):155–159. [DOI] [PubMed] [Google Scholar]
- 310. Gu WX, Du GG, Kopp P, Rentoumis A, Albanese C, Kohn LD, Madison LD, Jameson JL. The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology. 1995;136(7):3146–3153. [DOI] [PubMed] [Google Scholar]
- 311. Honegger A, Humbel RE. Insulin-like growth factors I and II in fetal and adult bovine serum. Purification, primary structures, and immunological cross-reactivities. J Biol Chem. 1986;261(2):569–575. [PubMed] [Google Scholar]
- 312. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. [DOI] [PubMed] [Google Scholar]
- 313. Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Currò N, Boschi A, Bernard M, von Arx G; European Group on Graves’ Orbitopathy . Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454–4463. [DOI] [PubMed] [Google Scholar]
- 314. Sisti E, Coco B, Menconi F, Leo M, Rocchi R, Latrofa F, Profilo MA, Mazzi B, Albano E, Vitti P, Marcocci C, Brunetto M, Marinò M. Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol. 2015;172(3):269–276. [DOI] [PubMed] [Google Scholar]
- 315. Kim JW, Han SH, Son BJ, Rim TH, Keum KC, Yoon JS. Efficacy of combined orbital radiation and systemic steroids in the management of Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):991–998. [DOI] [PubMed] [Google Scholar]
- 316. Marcocci C, Bartalena L, Panicucci M, Marconcini C, Cartei F, Cavallacci G, Laddaga M, Campobasso G, Baschieri L, Pinchera A. Orbital cobalt irradiation combined with retrobulbar or systemic corticosteroids for Graves’ ophthalmopathy: a comparative study. Clin Endocrinol (Oxf). 1987;27(1):33–42. [DOI] [PubMed] [Google Scholar]
- 317. Pinchera A, Marcocci C, Bartalena L, Panicucci M, Marconcini C, Lepri A, Cavallacci G, Cartei F, Laddaga M. Orbital cobalt radiotherapy and systemic or retrobulbar corticosteroids for Graves’ ophthalmopathy. Horm Res. 1987;26(1-4):177–183. [DOI] [PubMed] [Google Scholar]
- 318. Nakahara H, Noguchi S, Murakami N, Morita M, Tamaru M, Ohnishi T, Hoshi H, Jinnouchi S, Nagamachi S, Futami S. Graves ophthalmopathy: MR evaluation of 10-Gy versus 24-Gy irradiation combined with systemic corticosteroids. Radiology. 1995;196(3):857–862. [DOI] [PubMed] [Google Scholar]
- 319. Donaldson SS, Bagshaw MA, Kriss JP. Supervoltage orbital radiotherapy for Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1973;37(2):276–285. [DOI] [PubMed] [Google Scholar]
- 320. Donaldson SS, Bagshaw MA, Kriss JP. Letter: Orbital radiotherapy for the ophthalmopathy of Graves’s disease. N Engl J Med. 1974;290(14):805. [DOI] [PubMed] [Google Scholar]
- 321. Teng CS, Crombie AL, Hall R, Ross WM. An evaluation of supervoltage orbital irradiation for Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 1980;13(6):545–551. [DOI] [PubMed] [Google Scholar]
- 322. DeGroot LJ, Gorman CA, Pinchera A, Bartalena L, Marcocci C, Wiersinga WM, Prummel MF, Wartofsky L, Marocci C. Therapeutic controversies. Retro-orbital radiation and radioactive iodide ablation of the thyroid may be good for Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1995;80:339–340. [DOI] [PubMed] [Google Scholar]
- 323. Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, Dekker FW, Wiersinga WM. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89(1):15–20. [DOI] [PubMed] [Google Scholar]
- 324. Gorman CA, Garrity JA, Fatourechi V, Bahn RS, Petersen IA, Stafford SL, Earle JD, Forbes GS, Kline RW, Bergstralh EJ, Offord KP, Rademacher DM, Stanley NM, Bartley GB. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology. 2001;108(9):1523–1534. [DOI] [PubMed] [Google Scholar]
- 325. Kinyoun JL, Kalina RE, Brower SA, Mills RP, Johnson RH. Radiation retinopathy after orbital irradiation for Graves’ ophthalmopathy. Arch Ophthalmol. 1984;102(10):1473–1476. [DOI] [PubMed] [Google Scholar]
- 326. Robertson DM, Buettner H, Gorman CA, Garrity JA, Fatourechi V, Bahn RS, Petersen IA, Stafford SL, Earle JD, Forbes GS, Kline RW, Bergstralh EJ, Offord KP, Rademacher DM, Stanley NM, Bartley GB. Retinal microvascular abnormalities in patients treated with external radiation for graves ophthalmopathy. Arch Ophthalmol. 2003;121(5):652–657. [DOI] [PubMed] [Google Scholar]
- 327. Snijders-Keilholz A, De Keizer RJ, Goslings BM, Van Dam EW, Jansen JT, Broerse JJ. Probable risk of tumour induction after retro-orbital irradiation for Graves’ ophthalmopathy. Radiother Oncol. 1996;38(1):69–71. [DOI] [PubMed] [Google Scholar]
- 328. Schaefer U, Hesselmann S, Micke O, Schueller P, Bruns F, Palma C, Willich N. A long-term follow-up study after retro-orbital irradiation for Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 2002;52(1):192–197. [DOI] [PubMed] [Google Scholar]
- 329. Marcocci C, Bartalena L, Rocchi R, Marinò M, Menconi F, Morabito E, Mazzi B, Mazzeo S, Sartini MS, Nardi M, Cartei F, Cionini L, Pinchera A. Long-term safety of orbital radiotherapy for Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88(8):3561–3566. [DOI] [PubMed] [Google Scholar]
- 330. Wakelkamp IM, Tan H, Saeed P, Schlingemann RO, Verbraak FD, Blank LE, Prummel MF, Wiersinga WM. Orbital irradiation for Graves’ ophthalmopathy: is it safe? A long-term follow-up study. Ophthalmology. 2004;111(8):1557–1562. [DOI] [PubMed] [Google Scholar]
- 331. Prummel MF, Mourits MP, Blank L, Berghout A, Koornneef L, Wiersinga WM. Randomized double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet. 1993;342(8877):949–954. [DOI] [PubMed] [Google Scholar]
- 332. Wiersinga WM, Smit T, Schuster-Uittenhoeve AL, van der Gaag R, Koornneef L. Therapeutic outcome of prednisone medication and of orbital irradiation in patients with Graves’ ophthalmopathy. Ophthalmologica. 1988;197(2):75–84. [DOI] [PubMed] [Google Scholar]
- 333. Marcocci C, Bartalena L, Bogazzi F, Bruno-Bossio G, Lepri A, Pinchera A. Orbital radiotherapy combined with high dose systemic glucocorticoids for Graves’ ophthalmopathy is more effective than radiotherapy alone: results of a prospective randomized study. J Endocrinol Invest. 1991;14(10):853–860. [DOI] [PubMed] [Google Scholar]
- 334. Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366(21):2008–2016. [DOI] [PubMed] [Google Scholar]
- 335. Rath E, Zwerina J, Oppl B, Nell-Duxneuner V. Efficacy and safety of rituximab in rheumatic diseases. Wien Med Wochenschr. 2015;165(1-2):28–35. [DOI] [PubMed] [Google Scholar]
- 336. El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol. 2006;154(5):623–632. [DOI] [PubMed] [Google Scholar]
- 337. Hasselbalch HC. B-cell depletion with rituximab-a targeted therapy for Graves’ disease and autoimmune thyroiditis. Immunol Lett. 2003;88(1):85–86. [DOI] [PubMed] [Google Scholar]
- 338. Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, Guastella C, Avignone S, Pirola G, Ratiglia R, Beck-Peccoz P. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154(4):511–517. [DOI] [PubMed] [Google Scholar]
- 339. Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, Simonetta S, Guastella C, Pignataro L, Avignone S, Beck-Peccoz P. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100(2):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Pérez-Moreiras JV, Alvarez-López A, Gómez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthal Plast Reconstr Surg. 2014;30(2):162–167. [DOI] [PubMed] [Google Scholar]
- 342. van Steensel L, van Hagen PM, Paridaens D, Kuijpers RW, van den Bosch WA, Drexhage HA, Hooijkaas H, Dik WA. Whole orbital tissue culture identifies imatinib mesylate and adalimumab as potential therapeutics for Graves’ ophthalmopathy. Br J Ophthalmol. 2011;95(5):735–738. [DOI] [PubMed] [Google Scholar]
- 343. Bliddal S, Borresen SW, Feldt-Rasmussen U. Thyroid autoimmunity and function after treatment with biological antirheumatic agents in rheumatoid arthritis. Front Endocrinol (Lausanne). 2017;8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Kahaly GJ, Riedl M, König J, Pitz S, Ponto K, Diana T, Kampmann E, Kolbe E, Eckstein A, Moeller LC, Führer D, Salvi M, Curro N, Campi I, Covelli D, Leo M, Marinò M, Menconi F, Marcocci C, Bartalena L, Perros P, Wiersinga WM; European Group on Graves’ Orbitopathy (EUGOGO) . Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6(4):287–298. [DOI] [PubMed] [Google Scholar]
- 345. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. [published correction appears in Nat Rev Cancer 2009;9:22]. Nat Rev Cancer. 2008;8(12):915–928. [DOI] [PubMed] [Google Scholar]
- 346. Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001;86(7):2935–2941. [DOI] [PubMed] [Google Scholar]
- 347. Jenkins PJ. Acromegaly and cancer. Horm Res. 2004;62(Suppl 1):108–115. [DOI] [PubMed] [Google Scholar]
- 348. Baserga RPM, Yuan T. IGF-I receptor signaling in health and disease. In: LeRoith D, Zumkeller W, Baxter R, ed. Insulin-like Growth Factors. New York, NY: Eurekah.com and Kluwer Academic/Plenum Publishers; 2003:104–136. [Google Scholar]
- 349. Zheng H, Shen H, Oprea I, Worrall C, Stefanescu R, Girnita A, Girnita L. β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing’s sarcoma. Proc Natl Acad Sci USA. 2012;109(50):20620–20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, Bassi R, Abdullah R, Hooper AT, Koo H, Jimenez X, Johnson D, Apblett R, Kussie P, Bohlen P, Witte L, Hicklin DJ, Ludwig DL. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63(24):8912–8921. [PubMed] [Google Scholar]
- 351. Hansson HA, Petruson B, Skottner A. Somatomedin C in pathogenesis of malignant exophthalmos of endocrine origin. Lancet. 1986;1(8474):218–219. [DOI] [PubMed] [Google Scholar]
- 352. Matos K, Manso PG, Marback E, Furlanetto R, Alberti GN, Nosé V. Protein expression of VEGF, IGF-1 and FGF in retroocular connective tissues and clinical correlation in Graves’ ophthalmopathy. Arq Bras Oftalmol. 2008;71(4):486–492. [DOI] [PubMed] [Google Scholar]
- 353. Song D, Wang R, Zhong Y, Li W, Li H, Dong F. Locally produced insulin-like growth factor-1 by orbital fibroblasts as implicative pathogenic factor rather than systemically circulated IGF-1 for patients with thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):433–440. [DOI] [PubMed] [Google Scholar]
- 354. Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Smith TJ. Is IGF-I receptor a target for autoantibody generation in Graves’ disease? J Clin Endocrinol Metab. 2013;98(2):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Qu X, Wu Z, Dong W, Zhang T, Wang L, Pang Z, Ma W, Du J. Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy. Oncotarget. 2017;8(17):29501–29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Pritchard J, Tsui S, Horst N, Cruikshank WW, Smith TJ. Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves’ disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J Immunol. 2004;173(5):3564–3569. [DOI] [PubMed] [Google Scholar]
- 358. Suzuki S, Morimoto S, Fujishiro M, Kawasaki M, Hayakawa K, Miyashita T, Ikeda K, Miyazawa K, Yanagida M, Takamori K, Ogawa H, Sekigawa I, Takasaki Y. Inhibition of the insulin-like growth factor system is a potential therapy for rheumatoid arthritis. Autoimmunity. 2015;48(4):251–258. [DOI] [PubMed] [Google Scholar]
- 359. Tsushima H, Morimoto S, Fujishiro M, Yoshida Y, Hayakawa K, Hirai T, Miyashita T, Ikeda K, Yamaji K, Takamori K, Takasaki Y, Sekigawa I, Tamura N. Kinase inhibitors of the IGF-1R as a potential therapeutic agent for rheumatoid arthritis. Autoimmunity. 2017;50(5):329–335. [DOI] [PubMed] [Google Scholar]
- 360. Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62(2):199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]