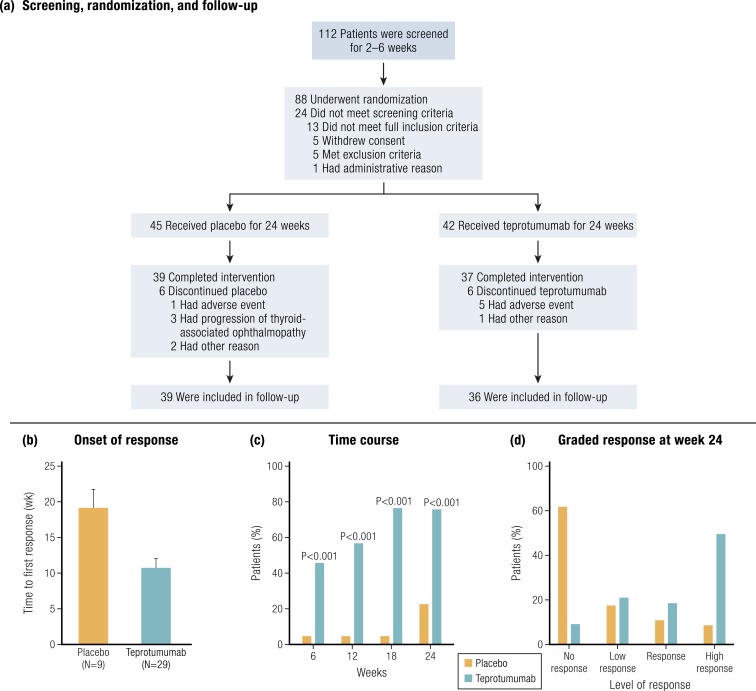
Figure 12.
Screening, randomization, response, and follow-up of patients’ participation in clinical trial RV001. (a) Patients meeting inclusion criteria entered the trial-screening process. At baseline, patients were randomized to receive active drug or placebo for the 24-week intervention phase. This was followed by a 1-year observation. (b) An analysis to first response. (c) The time course of patients meeting response criteria. (d) Responses are graded at week 24, where a high response indicates ≥3 mm proptosis and clinical activity score (CAS) reduced greater than or equal to three points on a seven-point scale. [Reproduced with permission from Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. Copyright Massachusetts Medical Society; © 2019 Illustration Presentation ENDOCRINE SOCIETY].