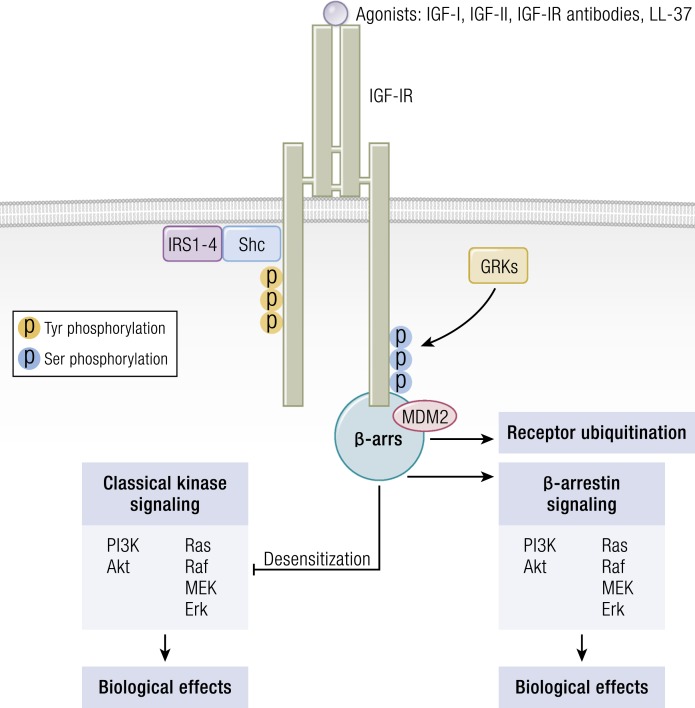
Figure 8.
The IGF-IR is now considered a functional RTK/GPCR hybrid, integrating canonical tyrosine kinase and GPCR signaling. Ligand binding activates classical kinase-dependent signaling pathways, as well as β-arrestin recruitment to GPCR kinase-dependent, phosphorylated serine residues within the C-tail of IGF-IR. β-Arrestin (β-ARR1) induces kinase desensitization and receptor ubiquitination while provoking kinase-independent signaling through the MAPK pathway. [Adapted from Worrall C, Nedelcu D, Serly J, Suleymanova N, Oprea I, Girnita A, Girnita L. Novel mechanisms of regulation of IGF-1R action: functional and therapeutic implications. Pediatr Endocrinol Rev. 2013;10(4):473–484; © 2019 Illustration Presentation ENDOCRINE SOCIETY].