Figure 3 – pADPr signaling in the nucleus.
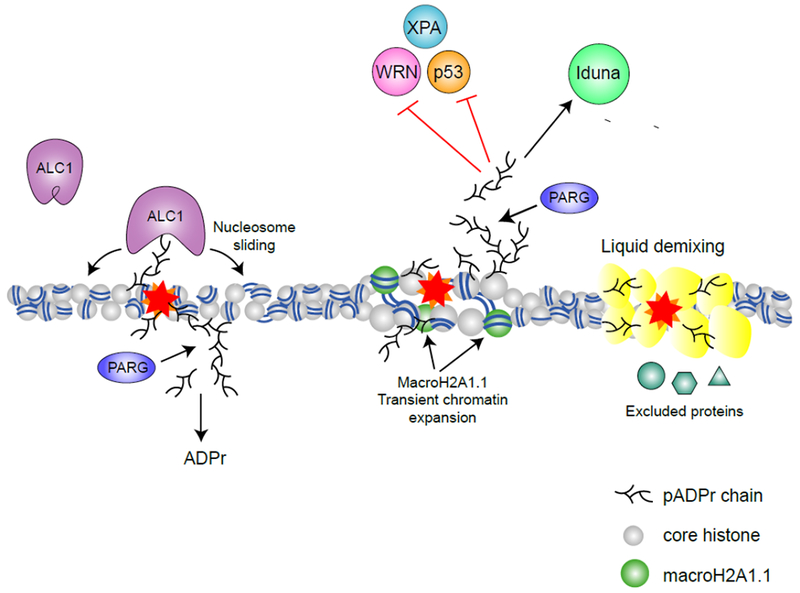
Through binding of pADPr, reader proteins are recruited and functionally regulated during DNA damage response. The chromatin remodeler ALC1 is recruited and activated by pADPr binding, promoting nucleosome sliding (63). MacroH2A1.1 is also recruited to DNA damage sites by pADPr and promotes transient chromatin expansion (32). pADPr binding can regulate protein functions either negatively (e.g. inhibiting nuclease activity of WRN (57), NER activity of XPA (58) and DNA binding activity of p53 (59)) or positively (e.g. activating the ATPase activity of ALC1 (63) and the E3 ligase activity of Iduna (73)). pADPr can also cause liquid demixing, forming a membrane-less organelle that retain some proteins but excludes others (76).
