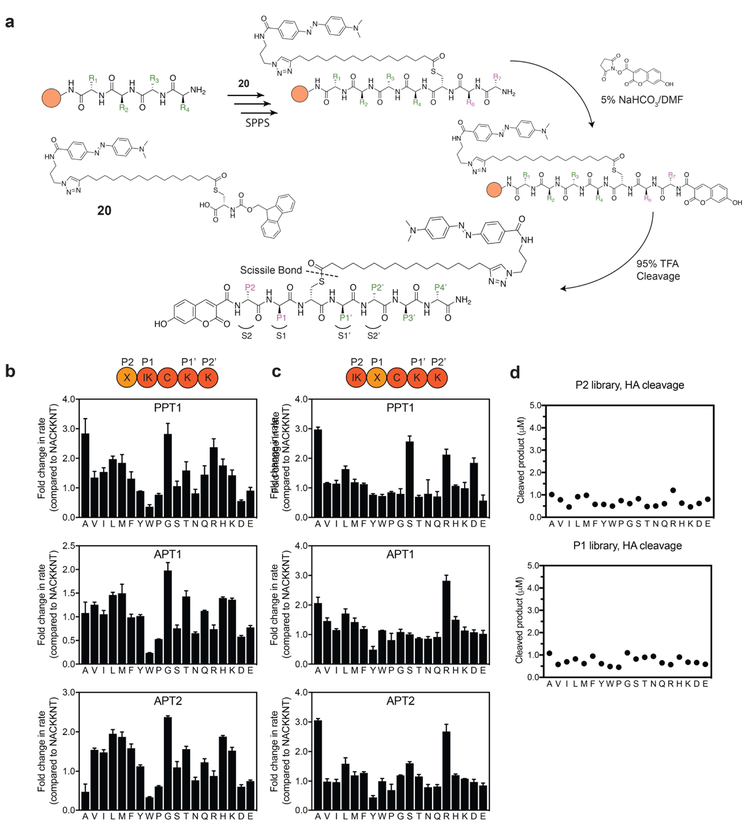
Figure 2: Positional scanning libraries highlight substrate specificities of APTs at positions P1 and P2.
a) Synthesis scheme for preparation of quenched fluorogenic substrates using solid phase peptide synthesis (SPPS). Peptide libraries were synthesized on a rink amide resin, the palmitoyl mimic containing DABCYL was synthesized as an Fmoc protected cysteine analogue (compound 20 or C20) and coupled instead of cysteine in the sequence. The N-terminus was capped with the flourophore 7-hydroxy-3-carboxycoumarin. Peptide positions spanning the thioester-containing cysteine analogue from the N-terminus side are referred to as P1, P2.., and from the C-terminus side as P1’, P2’... Libraries contain combinatorial mixtures (marked X) of all natural amino acids (excluding cysteine) at position P2 or position P1 and an isokinetic mixture of the same amino acids at the adjacent position (marked IK). b) Plots of fold-change in hydrolysis rates for each P2 scanning sublibrary relative to the reference peptide NAC20KKNT. Error bars represent S.D. of three replicates c). Same as in b) for the P1 positional scanning sublibraries. d) Effective concentration of each library was evaluated by chemical cleavage of the thioester bond by incubation with 5% HA for 30 minutes. Concentrations were estimated using a standard curve generated from non-quenched substrates. DBU = 1,8-Diazabicyclo(5.4.0)undec-7-ene. HA = hydroxylamine. See also figure S4.