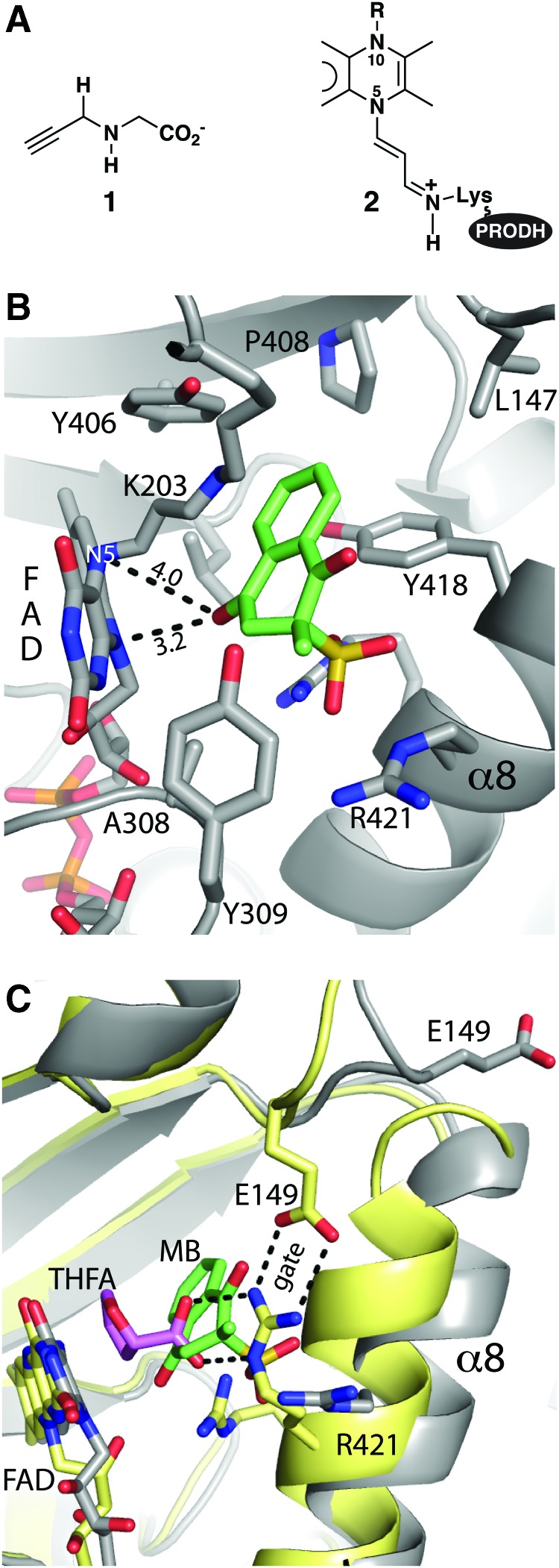
FIG. 4.
The quinone binding site of PRODH as seen in a structure of inactivated GsPutA complexed with MB. (A) Structures of (1) N-propargylglycine and (2) the covalently modified FAD resulting from inactivation by N-propargylglycine. In N-propargylglycine-inactivated GsPutA, Lys203 makes a covalent link with the FAD. (B) Interactions for MB (green) bound to inactivated GsPutA (PDB code 4NMF). The distances between MB and the N5 and N10 atoms of the FAD are indicated. (C) Comparison of the PRODH active sites of GsPutA-THFA (yellow protein, pink THFA) and inactivated GsPutA-MB (gray protein, green MB), highlighting the proximity of the proline and quinone sites and the structural differences involving α8 and Glu149. MB, menadione bisulfite. Figure adapted from Singh et al. (117). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars